Post-translational modifications of cancer immune checkpoints: mechanisms and therapeutic strategies
- PMID: 40629335
- PMCID: PMC12236040
- DOI: 10.1186/s12943-025-02397-5
Post-translational modifications of cancer immune checkpoints: mechanisms and therapeutic strategies
Abstract
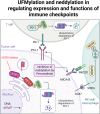
Immunotherapies, particularly immune checkpoint inhibitors (ICIs), have revolutionized cancer clinical management, but low response rates and treatment resistance remain challenging. Protein post-translational modifications (PTMs) are critical for governing protein expression, localization, functions, and interactions with other cellular molecules, which notably build up the diversity and complexity of the proteome. A growing body of evidence supports that PTMs influence immunotherapy efficacy and outcomes by post-translationally modulating the expression and functions of immune checkpoints. Therefore, understanding the PTM mechanisms that govern immune checkpoints is paramount for developing novel treatment strategies to improve immunotherapy efficacy and overcome resistance. This review provides an overview of the current comprehension of the regulatory mechanisms by which PTMs (glycosylation, phosphorylation, ubiquitination, acetylation, succinylation, palmitoylation, lactylation, O-GlcNAcylation, UFMylation, and neddylation) modulate immune checkpoints to unveil potential therapeutic targets. Moreover, this review discusses the potential of therapeutic strategies targeting PTMs of immune checkpoints, providing insights into the combination treatment with ICIs in maximizing the benefits of immunotherapy and overcoming resistance.
Keywords: Combination treatment; Immune checkpoints; Immunotherapy; Post-translational modifications; Treatment resistance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures








References
-
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223–49. - PubMed
-
- Miao C, Huang Y, Zhang C, Wang X, Wang B, Zhou X, Song Y, Wu P, Chen ZS, Feng Y. Post-translational modifications in drug resistance. Drug Resist Updat. 2025;78: 101173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

