Baseline functional connectivity of the basal forebrain-cortical circuit predict taVNS treatment response in primary insomnia: a randomized controlled trial and fMRI study
- PMID: 40629377
- PMCID: PMC12239473
- DOI: 10.1186/s12916-025-04126-7
Baseline functional connectivity of the basal forebrain-cortical circuit predict taVNS treatment response in primary insomnia: a randomized controlled trial and fMRI study
Abstract
Background: Dysfunctional basal forebrain (BF) connectivity contributes to primary insomnia (PI). This study investigated whether transcutaneous auricular vagus nerve stimulation (taVNS) modulates BF functional connectivity (FC) in patients with PI and whether baseline FC predicts taVNS treatment response.
Methods: Seventy patients with PI were randomized to real or sham taVNS for 4 weeks. Clinical assessments-including Pittsburgh Sleep Quality Index (PSQI], Insomnia Severity Index (ISI] and Zung's Self-Rating Anxiety (SAS], and Depression Scale (SDS)-and resting-state fMRI data were collected at baseline and after treatment. FC of the bilateral BF subregions (Ch_123, Ch_4) was analyzed, and pre-to-post intervention changes in FC and clinical scores were compared between groups. Baseline FC was used to predict treatment response using a support vector regression (SVR) model, validated on an independent dataset.
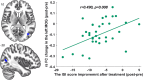
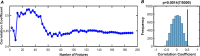
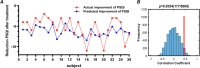
Results: Sixty-seven patients completed the study (33 real taVNS, 34 sham taVNS). Changes in clinical outcomes showed that real taVNS significantly reduce PSQI, ISI, and SAS scores compared to sham. FC analysis revealed reduced connectivity between bilateral BF and areas involved in visual (superior occipital gyrus, SOG; middle occipital gyrus, MOG; fusiform gyrus, FFG), somatosensory (supplementary motor area, SMA) cortex and medial prefrontal cortex (mPFC) after taVNS treatment. Reduced FC between bilateral BF and left MOG correlated positively with ISI improvement (r = 0.490, p = 0.008, Bonferroni correction). The SVR model effectively predicted treatment response based on BF-visual circuit connectivity (r = 0.520, p = 0.0014, 5000 permutation test) and generalized well to an independent dataset (r = 0.443, p = 0.0354, 5000 permutation test).
Conclusions: Our findings suggest that taVNS may alleviate symptoms of primary insomnia through modulation of basal forebrain connectivity with visual, sensorimotor, and medial prefrontal cortical regions. Preliminary investigations indicate that baseline functional connectivity in the BF-visual circuit could represent a candidate biomarker for taVNS response, potentially informing personalized treatment strategies.
Trial registration: The study was registered with the China Clinical Trial Registry (Clinical Trial No. ChiCTR1900022535).
Keywords: Functional connectivity of basal forebrain; Primary insomnia; Transcutaneous auricular vagus nerve stimulation; Treatment efficacy prediction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine approved the protocol (Approval No.: ZF2019-004-02). Written informed consent was obtained from all participants prior to study enrollment. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Transcutaneous Auricular Vagus Nerve Stimulation for Chronic Insomnia Disorder: A Randomized Clinical Trial.JAMA Netw Open. 2024 Dec 2;7(12):e2451217. doi: 10.1001/jamanetworkopen.2024.51217. JAMA Netw Open. 2024. PMID: 39680406 Free PMC article. Clinical Trial.
-
Transcutaneous auricular vagus nerve stimulation (taVNS) improves sleep quality in chronic insomnia disorder: A double-blind, randomized, sham-controlled trial.Sleep Med. 2025 Sep;133:106579. doi: 10.1016/j.sleep.2025.106579. Epub 2025 May 14. Sleep Med. 2025. PMID: 40398066 Clinical Trial.
-
Accelerated Transcutaneous Auricular Vagus Nerve Stimulation for Inpatient Depression and Anxiety: The iWAVE Open Label Pilot Trial.Neuromodulation. 2025 Jun;28(4):672-681. doi: 10.1016/j.neurom.2025.02.003. Epub 2025 Mar 19. Neuromodulation. 2025. PMID: 40117415 Clinical Trial.
-
Transcutaneous auricular vagus nerve stimulation for epilepsy.Seizure. 2024 Jul;119:84-91. doi: 10.1016/j.seizure.2024.05.005. Epub 2024 May 30. Seizure. 2024. PMID: 38820674
-
The efficacy and safety of transcutaneous auricular vagus nerve stimulation in the treatment of depressive disorder: A systematic review and meta-analysis of randomized controlled trials.J Affect Disord. 2023 Sep 15;337:37-49. doi: 10.1016/j.jad.2023.05.048. Epub 2023 May 23. J Affect Disord. 2023. PMID: 37230264
References
-
- Perlis ML, Posner D, Riemann D, Bastien CH, Teel J, Thase M. Insomnia. Lancet. 2022;400(10357):1047–60. - PubMed
-
- De Crescenzo F, D’Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, Kurtulmus A, Tomlinson A, Mitrova Z, Foti F, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. 2022;400(10347):170–84. - PubMed
-
- Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part I. Headache. 2016;56(1):71–8. - PubMed
-
- Rizzo P, Beelke M, De Carli F, Canovaro P, Nobili L, Robert A, Tanganelli P, Regesta G, Ferrillo F. Chronic vagus nerve stimulation improves alertness and reduces rapid eye movement sleep in patients affected by refractory epilepsy. Sleep. 2003;26(5):607–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

