CIITA-modified glioblastomas vaccinate and induce cross-protection against heterologous wild-type glioblastomas
- PMID: 40629415
- PMCID: PMC12239391
- DOI: 10.1186/s12967-025-06816-5
CIITA-modified glioblastomas vaccinate and induce cross-protection against heterologous wild-type glioblastomas
Abstract
Background: Glioblastoma (GBM) is a highly malignant brain tumor with poor outcome, despite current therapies. CIITA-modified tumor cells, capable of presenting antigens via MHC-II, show promise in eliciting effective antitumor immune responses. The efficacy of the CIITA-based vaccination strategy has been previously demonstrated using the GL261 murine GBM model. This study aimed at verifying whether a different GBM model, the CT-2A cells, more closely resembles human GBM, could follow the same rule and importantly share cross immunity with GL261.
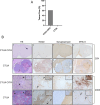
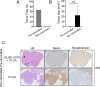
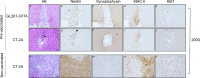
Methods: To assess tumor growth and immune response, immunocompetent mice were injected intracranially with either CT-2A or CIITA-modified CT-2A (CT-2A-CIITA) cells, and brain tissues were examined histologically. Additionally, mice vaccinated with GL261-CIITA cells were subsequently challenged with parental CT-2A cells in the opposite hemisphere, followed by immunohistochemical analysis of brain tissues.
Results: Results indicated that CT-2A-CIITA cells were either rejected or exhibited delayed tumor growth in immunocompetent mice. Furthermore, mice previously vaccinated with GL261-CIITA cells demonstrated efficient rejection of CT-2A tumors, suggesting the induction of cross-protective immunity. This immune response was associated with a shift from an immunosuppressive to an immunogenic tumor microenvironment, implying the development of adaptive immunity against shared tumor antigens.
Conclusions: In conclusion, these findings support the broader applicability of CIITA-based vaccination against GBM and provide the first evidence of shared immunogenic antigens between two distinct murine GBM models. This discovery opens avenues for identifying common tumor antigens through immunopeptidomics, which could inform future immunotherapeutic strategies for glioblastoma.
Keywords: CT-2A; Cross-protection; Glioblastoma; Immunotherapy; Tumor associated antigens; Vaccine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: All authors declare that no conflicts of interest exist.
Figures



Similar articles
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Upregulation of HLA-II related to LAG-3+CD4+ T cell infiltration is associated with patient outcome in human glioblastoma.Cancer Sci. 2024 May;115(5):1388-1404. doi: 10.1111/cas.16128. Epub 2024 Mar 13. Cancer Sci. 2024. PMID: 38480275 Free PMC article.
-
Single-dose radiotherapy is more effective than fractionation when combined with anti-PD-1 immunotherapy in glioblastoma.Sci Rep. 2025 Jul 2;15(1):22910. doi: 10.1038/s41598-025-06909-7. Sci Rep. 2025. PMID: 40596223 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Glioblastoma Immunotherapy: A Systematic Review of the Present Strategies and Prospects for Advancements.Int J Mol Sci. 2023 Oct 10;24(20):15037. doi: 10.3390/ijms242015037. Int J Mol Sci. 2023. PMID: 37894718 Free PMC article.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Preusser M, de Ribaupierre S, Wöhrer A, Erridge SC, Hegi M, Weller M, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70:9–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

