Immunological and Clinical Markers of Post-acute Sequelae of COVID-19: Insights from Mild and Severe Cases 6 Months Post-infection
- PMID: 40629965
- PMCID: PMC12238838
- DOI: 10.1002/eji.202551948
Immunological and Clinical Markers of Post-acute Sequelae of COVID-19: Insights from Mild and Severe Cases 6 Months Post-infection
Abstract
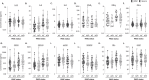
Post-acute sequelae of COVID-19 (PASC) are a complex clinical condition that requires a better understanding of its underlying biological mechanisms. In this study, we assessed hundreds of virological, serological, immunological, and tissue damage biomarkers in two cohorts of patients who had experienced either mild (n = 270) or severe (n = 188) COVID-19, 6 to 9 months post-initial infection, and in which 40% and 57.4% of patients, respectively, developed PASC. Blood analysis showed that the main differences observed in humoral, viral, and biological biomarkers were associated with the initial COVID-19 severity, rather than being specifically linked to PASC. However, patients with PASC displayed altered CD4+ and CD8+ memory T cell subsets, with higher cytokine-secreting cells and increased terminally differentiated CD45RA+ effector memory T cells (TEMRA). Elevated SARS-CoV-2-specific T cells responsive to nucleocapsid/membrane proteins with a TEMRA phenotype were also observed. A random forest model identified these features and initial symptom duration as top variables discriminating PASC, achieving over 80% classification accuracy.
Keywords: SARS‐CoV‐2; T cell phenotyping; biological biomarkers; humoral; postacute sequelae of COVID‐19 (PASC); virological.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Anon. APCOVID‐19 : étude Nationale Sur La Prévalence Et L'impact De L'affection post‐COVID‐19 Available at: [Accessed 2024], https://www.santepubliquefrance.fr/etudes‐et‐enquetes/apcovid‐19‐etude‐n....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

