Acellular Pertussis Vaccines Induce CD8+ and CD4+ Regulatory T Cells That Suppress Protective Tissue-Resident Memory CD4+ T Cells, in Part via IL-10
- PMID: 40629997
- PMCID: PMC12238839
- DOI: 10.1002/eji.202451630
Acellular Pertussis Vaccines Induce CD8+ and CD4+ Regulatory T Cells That Suppress Protective Tissue-Resident Memory CD4+ T Cells, in Part via IL-10
Abstract
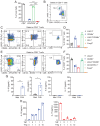
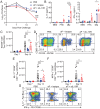
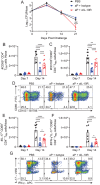
Tissue-resident memory T (TRM) cells play a key role in sustained protective immunity against Bordetella pertussis infection of the nasal mucosa. Current alum-adjuvanted acellular pertussis (aP) vaccines protect against severe pertussis disease but fail to prevent nasal infection with B. pertussis. Here we demonstrate that immunization of mice with an aP vaccine failed to generate respiratory TRM cells, but did induce antigen-specific CD4+ Treg cells that expressed Foxp3, CD49b, PD-1 and LAG-3, and CD8+ Treg cells that expressed CD122, PD-1, and IL-10. B. pertussis-specific CD4+ and CD8+ T cell lines established from aP-immunized mice expressed the regulatory markers and suppressed activation of Th1 and Th17 cells. Blockade of IL-10 signaling during aP immunization or B. pertussis challenge promoted the induction of IL-17-secreting CD4+ TRM responses and enhanced bacterial clearance from the nose. Addition of the adjuvant LP-GMP, comprising TLR2 and STING agonists, to the aP vaccine and delivery by the nasal route promoted the induction of antigen-specific IL-17-producing CD4+ TRM cells and enhanced vaccine efficacy. Our findings demonstrate that aP vaccines suppress the induction of protective TRM cells in part through the induction of CD4+ and CD8+ Treg cells, which can be overcome using a potent adjuvant and delivery of the vaccine intranasally.
Keywords: IL‐10; TRM cell; Th17 cell; Treg cell; pertussis vaccine.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
Kingston Mills is the co‐founder of a Start‐up company and has collaborative research funding from and acts as a consultant to Pharmaceutical and Biotech companies. Kingston Mills, Béré Diallo, Caitlín Ní Chasaide, and Pauline Schmitt are inventors on a patent application around a novel vaccine adjuvant.
Figures



Similar articles
-
Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis.Emerg Microbes Infect. 2019;8(1):169-185. doi: 10.1080/22221751.2018.1564630. Emerg Microbes Infect. 2019. PMID: 30866771 Free PMC article.
-
Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells.Mucosal Immunol. 2018 Nov;11(6):1763-1776. doi: 10.1038/s41385-018-0080-x. Epub 2018 Aug 20. Mucosal Immunol. 2018. PMID: 30127384
-
A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against Bordetella pertussis, Including Pertactin Deficient Strains.Front Cell Infect Microbiol. 2019 Apr 26;9:125. doi: 10.3389/fcimb.2019.00125. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31106160 Free PMC article.
-
Acellular vaccines for preventing whooping cough in children.Cochrane Database Syst Rev. 2012 Mar 14;(3):CD001478. doi: 10.1002/14651858.CD001478.pub5. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Sep 17;(9):CD001478. doi: 10.1002/14651858.CD001478.pub6. PMID: 22419280 Updated.
-
Adaptive immune response to bordetella pertussis during vaccination and infection: emerging perspectives and unanswered questions.Expert Rev Vaccines. 2024 Jan-Dec;23(1):705-714. doi: 10.1080/14760584.2024.2383745. Epub 2024 Jul 24. Expert Rev Vaccines. 2024. PMID: 39037200 Free PMC article. Review.
References
-
- Smout E., Mellon D., and Rae M., “Whooping Cough Rises Sharply in UK and Europe,” Bmj 385 (2024): q736. - PubMed
-
- Klein N. P., Bartlett J., Rowhani‐Rahbar A., Fireman B., and Baxter R., “Waning Protection After Fifth Dose of Acellular Pertussis Vaccine in Children,” New England Journal of Medicine 367 (2012): 1012–1019. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

