Retention and characteristics associated with remote questionnaire completion in a general population cohort study: the project baseline health study
- PMID: 40630050
- PMCID: PMC12235916
- DOI: 10.3389/fdgth.2025.1520132
Retention and characteristics associated with remote questionnaire completion in a general population cohort study: the project baseline health study
Abstract
Objective: To evaluate remote participant engagement in a clinical study over time, based on data from the Project Baseline Health Study (PBHS), a hybrid in-person and virtual study.
Methods: The PBHS enrolled 2,502 adult US residents from March 3, 2017 to April 26, 2019, with a ≤5-year follow-up. We summarized 4-year retention and rates of longitudinal patient-reported outcome survey completion. We investigated participant characteristics for their associations with quarterly remote survey completion using regression models.
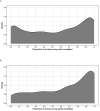
Results: Of the total participants (N = 2,502), 94% remained enrolled after 4 years and 60% completed all annual visits; 2,490 participants stayed enrolled for at least one quarter. The median (IQR) number of remote electronic survey sets completed was 8 (3-12), of a possible 16. Age [odds ratio (OR), >70 vs. ≤30 years: 2.56; 95% CI: 2.24-2.94] and education (OR, advanced degree vs. ≤high school: 1.36; 95% CI: 1.22-1.52) were positively associated with remote survey completion. Participants with lower odds of completion were Black (OR vs. White: 0.73; 95% CI: 0.67-0.80), Hispanic (OR vs. non-Hispanic: 0.84; 95% CI: 0.77-0.93), or had at least mild symptoms of depression (OR vs. without: 0.90; 95% CI: 0.84-0.96) or anxiety (OR vs. without: 0.84; 95% CI: 0.78-0.90).
Conclusions: Overall, 94% of PBHS participants remained enrolled after four years. Age, race, ethnicity, income, education, and symptomatic depression/anxiety were significantly associated with longitudinal remote questionnaire completion. These findings on engagement over time may inform future longitudinal study design.
Clinical trial registration: Clinicaltrials.gov, identifier (NCT03154346).
Keywords: PBHS; cohort study; participant retention; patient-reported outcomes; project baseline health study; remote study; research participant engagement; social determinants of health.
© 2025 Carroll, Faheem, Bouteiller, Hernandez, Mahaffey, Mega, Pagidipati, Schaack, Shah, Shashidhar, Swope, Williams, Plowman, Simard, Short and Sullivan.
Conflict of interest statement
The authors declare that the Project Baseline Health Study and this analysis received funding from Verily Life Sciences, South San Francisco, California. The funder had the following involvement in the study: responsibility for data collection. MC, SF, JB, JM, RP, ES, SarS, ShaS report employment and equity ownership in Verily Life Sciences. KM reports research grants from Verily, American Heart Association, Apple Inc., Bayer, the California Institute of Regenerative Medicine, Eidos, Gilead, Idorsia, Johnson & Johnson, Luitpold, Pac-12, Precordior, Sanifit; consulting fees from Amgen, Applied Therapeutics, BMS, BridgeBio, Elsevier, Lexicon, Moderna, Sanofi; equity ownership in Precordior, Regencor. NJP reports the following research support from Alnylam, Amgen, Bayer, Boehringer Ingelheim, Eggland's Best, Eli Lilly, Novartis, Novo Nordisk, Merck. Consultation/Advisory Panels for Bayer, Boehringer Ingelheim, CRISPR Therapeutics, Eli Lilly, Esperion, AstraZeneca, Merck, Novartis, and Novo Nordisk. Executive Committee member for trials sponsored by Novo Nordisk and by Amgen. DSMB for trials sponsored by J+J and Novartis. Medical advisory board for Miga Health.
Figures

References
-
- Klein D, Montgomery A, Begale M, Sutherland S, Sawyer S, McCauley JL, et al. Building a digital health research platform to enable recruitment, enrollment, data collection, and follow-up for a highly diverse longitudinal US cohort of 1 million people in the all of US research program: design and implementation study. J Med Internet Res. (2025) 27:e60189. 10.2196/60189 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

