A naphthalimide derivative exerts potent antiplatelet and antithrombotic activities without a bleeding tendency
- PMID: 40630131
- PMCID: PMC12234328
- DOI: 10.3389/fphar.2025.1541255
A naphthalimide derivative exerts potent antiplatelet and antithrombotic activities without a bleeding tendency
Abstract
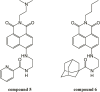
Background: Bleeding is the inherent adverse effect of antiplatelet drugs that has limited their use in the prevention of secondary heart attack and stroke. Thus, finding a novel antiplatelet drug with antithrombotic activities while preserving hemostatic function remains a crucial issue. Here, we screened naphthalimide derivatives that we previously synthesized and identified a novel derivative compound 6, which has a more potent antithrombotic effect and has no effect on bleeding cessation. This study is aimed to determine the antiplatelet mechanism of compound 6 and further test whether compound 6 is a safer and more potent antithrombotic agent.
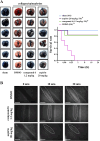
Methods: Platelet aggregation, flow cytometry and immunoblotting were used to determine the in vitro antiplatelet effect of compound 6. The study of thrombus formation of mesenteric venules in mice was used to evaluate the antithrombotic effect of compound 6.
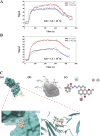
Results: Compound 6 selectively inhibited collagen-mediated platelet aggregation and markedly prevented thrombus formation without bleeding tendency. Compound 6 also inhibited glycoprotein VI (GPVI) downstream signaling, such as Fyn and Lyn, phospholipase C gamma 2, protein kinase C. Moreover, a surface plasmon resonance assay indicated that compound 6 may directly bind to GPVI, thereby interrupting the interaction of collagen and GPVI. Compound 6 also effectively attenuates collagen-induced granule release, calcium mobilization, and GPIIbIIIa activation.
Conclusion: These findings indicate that compound 6 can selectively inhibit GPVI, eventually suppressing platelet activation and thrombus formation while preserving hemostasis. Compound 6 is a GPVI antagonist and safe antiplatelet agent. Compound 6 also has therapeutic potential for treating cardiovascular diseases.
Keywords: GPVI; antiplatelet agent; naphthalimide derivative; platelet activation; thrombus formation.
Copyright © 2025 Lu, Li, Shih, Chen, Chen, Kao, Liu, Wang, Peng and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bellier B., Mccort-Tranchepain I., Ducos B., Danascimento S., Meudal H., Noble F., et al. (1997). Synthesis and biological properties of new constrained CCK-B antagonists: discrimination of two affinity states of the CCK-B receptor on transfected CHO cells. J. Med. Chem. 40, 3947–3956. 10.1021/jm970439a - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

