Randomized, Positive-Controlled Study on the Efficacy and Safety of Oral Polysaccharide-Iron Complex Therapy in Patients on Hemodialysis
- PMID: 40630322
- PMCID: PMC12231038
- DOI: 10.1016/j.ekir.2025.03.017
Randomized, Positive-Controlled Study on the Efficacy and Safety of Oral Polysaccharide-Iron Complex Therapy in Patients on Hemodialysis
Abstract
Introduction: The IHOPE study evaluated the efficacy and safety of oral iron polysaccharide complex versus i.v. iron in Chinese patients on hemodialysis (HD).
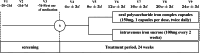
Methods: This randomized, open-label, noninferiority trial enrolled adults (aged 18-75 years) on maintenance HD (MHD) with hemoglobin of 100 to 130 g/l, transferrin saturation (TSAT) of 20% to 50%, or serum ferritin of 100 to 500 μg/l. Patients were randomized 1:1 to receive oral iron polysaccharide complex (150 mg twice daily) or i.v. iron sucrose (100 mg biweekly) for 24 weeks. The primary outcome was TSAT at 12 weeks. Noninferiority margin was set at 7% with significance at 0.05.
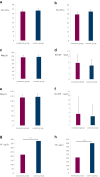
Results: Among 193 participants (mean age: 55.3 years, 62.2% male), 12-week TSAT was 32.3% (95% CI: 29.39%-35.19%) in the oral group versus 33.4% (95% CI: 30.95%-35.77%) in the i.v. group, demonstrating noninferiority. At 24 weeks, TSAT remained noninferior (29.4% vs. 32.6%), and hemoglobin levels were comparable (114.6 vs. 117.4 g/l; P = 0.166). Adverse events occurred in 51.0% of oral and 47.4% of i.v. group patients, with serious adverse events in 14.6% and 13.4%, respectively.
Conclusion: Oral iron polysaccharide complex demonstrated noninferiority to i.v. iron sucrose for maintaining iron status in patients on HD, with comparable safety profiles.
Keywords: hemodialysis; oral iron supplements; renal anemia.
© 2025 Published by Elsevier, Inc., on behalf of the International Society of Nephrology.
Figures

References
-
- Chinese Experts Group of the Guideline for the Management of ‘CKD-PeriDialysis,’ Chinese Non-government Medical Institutions Association Chinese clinical practice guideline for the management of “CKD-PeriDialysis”-the periods prior to and in the early-stage of initial dialysis. Kidney Int Rep. 2022;7:S531–S558. doi: 10.1016/j.ekir.2022.10.001. - DOI - PMC - PubMed
-
- Chinese Medical Association Nephrology Physician Branch Renal Anemia Guideline Working Group Chinese clinical practice guidelines for the diagnosis and treatment of renal anemia. Natl Med J China. 2021;101:1463–1502. doi: 10.3760/cma.j.cn112137-20210201-00309. - DOI
-
- Lu R., Zhang X., Cai X., et al. Efficacy and safety of polysaccharide iron complex capsules compared with iron sucrose in hemodialysis patients: study protocol for a randomized, open-label, positive control, multicenter trial (IHOPE) Trials. 2021;22:691. doi: 10.1186/s13063-021-05663-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

