Dose-Dependent Efficacy and Safety of Tirzepatide for Weight Loss in Non-diabetic Adults With Obesity: A Systematic Review and Meta-analysis of Randomized Controlled Trials
- PMID: 40630338
- PMCID: PMC12234836
- DOI: 10.7759/cureus.85531
Dose-Dependent Efficacy and Safety of Tirzepatide for Weight Loss in Non-diabetic Adults With Obesity: A Systematic Review and Meta-analysis of Randomized Controlled Trials
Abstract
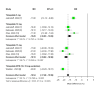
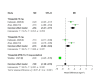
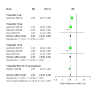
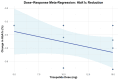
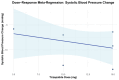
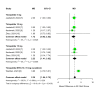
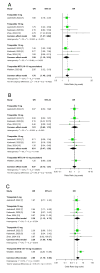
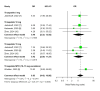
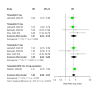
Tirzepatide, a dual glucose-dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonist, has shown promising weight-loss effects in previous trials. However, its dose-dependent efficacy and safety in obese adults without diabetes have not been fully defined. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) evaluating tirzepatide in obese adults without diabetes. PubMed, Cochrane CENTRAL, and ClinicalTrials.gov were searched from January 2020 to April 2025. Eligible studies compared tirzepatide (5-15 mg or maximum tolerated dose (MTD)) to a placebo over a minimum duration of 52 weeks. The primary outcome was the percent change in body weight. Secondary outcomes included changes in body mass index (BMI), waist circumference, hemoglobin A1c (HbA1c), systolic blood pressure (SBP), quality-of-life scores (Short-Form Health Survey Version 2 (SF-36v2) and the Impact of Weight on Quality of Life-Lite Clinical Trials version (IWQOL-Lite-CT)), and achievement of categorical weight-loss thresholds. Safety outcomes included the incidence of adverse events. Data were pooled using fixed- or random-effects models, and dose-response meta-regression was performed. Four RCTs (n = 3,553 participants) met the inclusion criteria. Tirzepatide significantly reduced the percentage body weight compared to placebo (mean difference -16.54%; 95% CI -17.48 to -15.59; p < 0.00001), with a clear dose-response relationship (meta-regression β = -0.72% per 1 mg increase; p = 0.0014). Participants receiving tirzepatide were markedly more likely to achieve clinically meaningful weight-loss thresholds, such as ≥15% (OR 23.25; 95% CI 18.06-29.94). Tirzepatide also improved BMI (-7.09 kg/m²), waist circumference (-12.77 cm), HbA1c (-0.42%), and quality-of-life scores. Gastrointestinal adverse events, including nausea (OR 4.20), vomiting (OR 6.93), and diarrhea (OR 3.80) were more frequent with tirzepatide; however, the rate of serious adverse events was comparable to placebo (OR 0.97). Sensitivity analyses confirmed the reliability of the findings. In conclusion, tirzepatide produces substantial, dose-dependent weight loss and improves metabolic and quality-of-life outcomes in obese adults without diabetes. Despite a higher incidence of gastrointestinal side effects, the lack of increased serious adverse events supports its favorable risk-benefit profile for long-term obesity treatment.
Keywords: dose response; dual incretin therapy; gip/glp-1 agonist; meta-analysis; meta-regression; non-diabetic adults; obesity treatment; systematic review; tirzepatide; weight loss therapy.
Copyright © 2025, Kasagga et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures









References
-
- Obesity and overweight. [ May; 2025 ]. 2025. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
-
- Obesity as a disease: The Obesity Society 2018 position statement. Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity (Silver Spring) 2019;27:7–9. - PubMed
-
- 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): indications for metabolic and bariatric surgery. Eisenberg D, Shikora SA, Aarts E, et al. Surg Obes Relat Dis. 2022;18:1345–1356. - PubMed
-
- Once-weekly semaglutide in adults with overweight or obesity. Wilding JP, Batterham RL, Calanna S, et al. N Engl J Med. 2021;384:989–1002. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
