Malignancy in neuromuscular patients on chronic IVIG
- PMID: 40630912
- PMCID: PMC12234317
- DOI: 10.3389/fneur.2025.1571160
Malignancy in neuromuscular patients on chronic IVIG
Abstract
Background: Intravenous immunoglobulin (IVIG) has been widely used to treat immune-mediated neuromuscular disorders. The relationship between IVIG and cancer is unclear. Preclinical studies have suggested that IVIG may influence cellular mechanisms pertinent to cancer development. This hypothesis is supported by clinical evidence, predominantly through case reports, although these findings are limited in scope and methodological rigor.
Methods: A retrospective review was conducted on patients receiving chronic IVIG treatment for Myasthenia Gravis (MG) and chronic immune demyelinating polyradiculoneuropathy (CIDP), at our tertiary medical center between 2000 and 2023.
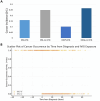
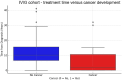
Results: We identified 436 patients with MG and 102 patients with CIDP. Patients were divided into IVIG and non-IVIG treated in each of the disease groups. Seventy-five and 64 patients received IVIG in MG and CIDP groups, respectively. Cancer incidence was counted if it appeared at least 1 year after the diagnosis of MG or CIDP. In the MG group, cancer incidence was 12 / 75 (16%) among IVIG-treated patients compared to 87/350 (25%) in the MG-non-IVIG group (p = 0.09). Excluding mild MG cases, who were treated only by pyridostigmine, cancer incidence in the MG-non-IVIG group increased to 72/250 (28.8%), significantly higher than in the IVIG-MG group, p = 0.01. For CIDP patients receiving IVIG, incidence of cancer was 6/59 (10%), while in the non-IVIG-treated CIDP group, it was 9/34 (26%) (p = 0.03). Analyzing all IVIG-treated patients together (of both disease groups) showed a negative correlation between "time on IVIG treatment" and cancer (p = 0.001). Using logistic regression, we observed a negative coefficient for IVIG exposure (-0.03, p = 0.004), indicating that an increase in time from diagnosis is associated with a decreased likelihood of developing cancer in the IVIG-treated group.
Conclusion: Chronic IVIG therapy may be associated with a reduced incidence of cancer, particularly among patients with CIDP. Additionally, we found a negative correlation between duration of IVIG treatment and cancer incidence, suggesting a potential protective effect of long-term IVIG exposure. To our knowledge, this is the first study to explore the relationship between sustained IVIG therapy and long-term cancer risk in neuromuscular autoimmune diseases.
Keywords: CIDP; IVIG; Myasthenia Gravis; autoimmune disorders; cancer.
Copyright © 2025 Khateb, Bahous, Abu Zant and Shelly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD010369. doi: 10.1002/14651858.CD010369.pub2. Cochrane Database Syst Rev. 2017. PMID: 28084646 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy.Cochrane Database Syst Rev. 2017 May 8;5(5):CD003280. doi: 10.1002/14651858.CD003280.pub5. Cochrane Database Syst Rev. 2017. PMID: 28481421 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome.Cochrane Database Syst Rev. 2022 Mar 11;3(3):CD013130. doi: 10.1002/14651858.CD013130.pub2. Cochrane Database Syst Rev. 2022. PMID: 35274741 Free PMC article.
References
LinkOut - more resources
Full Text Sources

