Virology Outcomes of Tenofovir-lamivudine-dolutegravir in Treatment-naïve and Virologically Suppressed Individuals Switching From an NNRTI-based Regimen: An Observational Analysis at 13 Sites
- PMID: 40630936
- PMCID: PMC12236153
- DOI: 10.1093/ofid/ofaf270
Virology Outcomes of Tenofovir-lamivudine-dolutegravir in Treatment-naïve and Virologically Suppressed Individuals Switching From an NNRTI-based Regimen: An Observational Analysis at 13 Sites
Abstract
Background: Tenofovir/lamivudine/dolutegravir (TLD) is widely prescribed worldwide. We report virologic and resistance outcomes for patients initiating or switching to TLD.
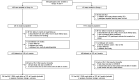
Methods: A prospective observational study was performed at 13 AIDS Clinical Trials Group sites in 6 President's Emergency Plan for AIDS Relief-supported countries coincident with TLD rollout. This report includes results from 2 groups: group 1 (Gp1) were virally suppressed on nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy (ART) and group 2 (Gp2) were ART-naïve at TLD initiation. The primary objective was to estimate the proportions of participants with HIV-1 RNA ≤1000 copies/mL and frequency of dolutegravir resistance mutations 6 months after TLD initiation.
Results: From October 2019 through July 2022, we enrolled 425 participants in Gp1 and 179 in Gp2. Two in Gp1 (0.5%) and 3 in Gp2 (1.7%) discontinued TLD by 6 months due to adverse events considered related to TLD (n = 4) and participant decision (n = 1). Ninety-three percent of participants in Gp1 and 92% in Gp2 who were still on TLD had a 6-month plasma HIV-1 RNA. Plasma HIV-1 RNA ≤1000, ≤ 200, and <50 copies/mL was achieved in 99%, 98%, and 96% in Gp1 and in 90%, 87%, and 85% in Gp2, respectively. A new integrase mutation (T97A/T) was observed in 1 participant in Gp1 and none in Gp2.
Conclusions: TLD was well tolerated and achieved or maintained viral suppression (≤1000 copies/mL) in 90% of ART-naïve and 99% of participants with preswitch viral suppression. An emerging integrase strand transfer inhibitor mutation of uncertain significance was detected in only 1 participant. These data support early tolerability, virologic efficacy, and rare integrase strand transfer inhibitor resistance emergence with TLD transition or initiation in programmatic settings.
Keywords: ART; Africa region; LMIC; observational prospective study; viral suppression.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential Conflicts of Interest. C.F. has been a paid consultant in the past 3 years to Gilead Sciences, Merck, and ViiV Healthcare. Other authors do not have conflict of interest with regards to this work.
References
-
- Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. - PubMed
-
- Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. - PubMed
-
- Calmy A, Sanchez TT, Kouanfack C, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020; 7:e677–87. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources