Applications and insights from continuous dengue virus infection in a stable cell line
- PMID: 40630949
- PMCID: PMC12234473
- DOI: 10.3389/fimmu.2025.1618650
Applications and insights from continuous dengue virus infection in a stable cell line
Abstract
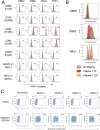
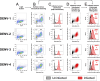
Dengue is caused by the four serotypes of dengue virus (DENV-1-4) and poses a significant global public health challenge, with an estimated 100-400 million infections annually. Severe dengue manifestations, such as Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS), are influenced by immune responses, particularly during secondary infections with different serotypes. Antibody-dependent enhancement (ADE) of DENV infection is a critical mechanism in dengue immunopathogenesis, underscoring the need for comprehensive evaluation of antibody responses. Traditional cell lines used for DENV propagation exhibit variability and present logistical challenges for assessing non-neutralizing antibody functions. Here, we report the establishment of a stable CEM-NKR cell line expressing DC-SIGN, designated CEM2001, capable of supporting continuous infection with all four DENV serotypes. These cell lines allow for continuous DENV infection, enabling detailed immunoassays to evaluate serotype-specific and cross-reactive non-neutralizing antibody responses. Our approach offers a significant advancement in dengue research, providing a consistent and reliable system to study DENV immune responses and supporting future efforts to develop and evaluate dengue therapeutics and vaccines.
Keywords: CEM.NKR; DC-SIGN; DENV; dengue; dengue therapeutics; dengue vaccines; immunoassay; serotype.
Copyright © 2025 Morwitzer, Zheng, Friberg and Currier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Improving data for dengue. Available online at: https://www.who.int/activities/improving-data-for-dengue (Accessed July 30, 2024).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

