Safety and efficacy of empagliflozin in heart failure among patients with a history of valvular heart disease: Insights from EMPEROR-Pooled
- PMID: 40631012
- PMCID: PMC12230564
- DOI: 10.1016/j.xjon.2025.03.018
Safety and efficacy of empagliflozin in heart failure among patients with a history of valvular heart disease: Insights from EMPEROR-Pooled
Abstract
Background: Valvular heart disease (VHD)-associated heart failure (HF) remains an important and growing cause of morbidity and mortality. There are no contemporary data on the efficacy and safety of SGLT2 inhibitors in patients with a history of VHD.
Methods: The EMPEROR-Pooled trial analyzed 9718 patients with HF who were enrolled in the randomized trials of empagliflozin versus placebo in HF with reduced left ventricular ejection fraction (HfrEF; EMPEROR-Reduced) and HF with preserved left ventricular ejection fraction (HFpEF; EMPEROR-Preserved). These trials evaluated a primary outcome of time to first HF hospitalization or cardiovascular death. Here we analyze outcomes of the EMPEROR-Pooled patients according to the presence and etiology of VHD history.
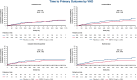
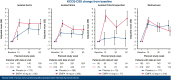
Results: Of the 9717 patients enrolled in EMPEROR-Pooled with available data, 1484 (15.3%) had a history of VHD. Of the patients with VHD history, a history of isolated mitral disease (39.2%) was the most common subtype. In patients randomized to placebo, the risk of the primary outcome was higher among patients with VHD history (hazard ratio [HR], 1.30; 95% confidence interval [CI], 1.10-1.53; P < .01), and particularly those with a history of multivalvular disease (HR, 1.51; 95% CI, 1.13-2.04; P < .01) compared with no valvular disease. No heterogeneity was introduced by VHD history with respect to the efficacy of empagliflozin on all major clinical outcomes evaluated in EMPEROR-Pooled (P interaction > .05).
Conclusions: We present the first large analysis of SGLT2i (empagliflozin) use in HF patients by history of VHD. Although VHD history was associated with worse outcomes in HF patients, empagliflozin demonstrated consistent safety, efficacy, and patient-reported outcomes across all categories of VHD history.
Keywords: co-transporter 2 inhibitors; heart failure; sodium-glucose; valvular heart disease.
© 2025 The Author(s).
Conflict of interest statement
N.K.D. reported statistical support from Amarin, Boehringer Ingelheim, Eli Lilly, Lexicon Pharmaceuticals, and Sanofi. E.P. and T.G. are employees of Boehringer Ingelheim. S.V. is supported by the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada; holds the Tier 1 Canada Research Chair in Cardiovascular Surgery; and has received speaking honoraria and/or consulting fees from Abbott, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge Translation Research Group, Eli Lilly, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, and TIMI. All other authors reported no conflicts of interest. The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Figures

References
-
- Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

