This is a preprint.
PCNA is a Nucleotide Exchange Factor for the Clamp Loader ATPase Complex
- PMID: 40631102
- PMCID: PMC12236598
- DOI: 10.1101/2025.07.02.662830
PCNA is a Nucleotide Exchange Factor for the Clamp Loader ATPase Complex
Abstract
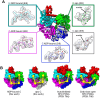
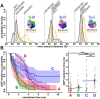
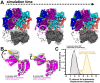
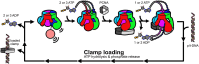
All life requires loading ring-shaped sliding clamp protein complexes onto DNA. The sliding clamp loader is a conserved AAA+ ATPase that binds the sliding clamp, opens the ring, and places it onto DNA. While recent structural work on both the canonical and 'alternative' clamp loaders has shed light into how these machines perform their task once, it remains unclear how clamp loaders are recycled to load multiple sliding clamps. Here, we present structures of the Saccharomyces cerevisiae clamp loader Replication Factor C (RFC) in absence of sliding clamp or supplemented nucleotide. Our structures indicate that RFC holds onto ADP tightly in at least two of its four ATPase active sites, suggesting that nucleotide exchange is regulated. Our molecular dynamics simulations and biochemical data indicate that binding of the sliding clamp PCNA causes rapid exchange of tightly bound ADP. Our data suggests that PCNA acts as a nucleotide exchange factor by prying apart adjacent subunits, providing a pathway for ADP release. We propose that, by using its own substrate as a nucleotide exchange factor, RFC excludes off-pathway states that would arise from binding DNA prior to PCNA.
Figures



Similar articles
-
Structure of the human CTF18-RFC clamp loader bound to PCNA.bioRxiv [Preprint]. 2025 Jul 24:2024.05.08.593111. doi: 10.1101/2024.05.08.593111. bioRxiv. 2025. PMID: 40777363 Free PMC article. Preprint.
-
A non-catalytic role for RFC in PCNA-mediated processive DNA synthesis.bioRxiv [Preprint]. 2025 Aug 12:2025.08.08.669392. doi: 10.1101/2025.08.08.669392. bioRxiv. 2025. PMID: 40832337 Free PMC article. Preprint.
-
Cryo-EM structures reveal high-resolution mechanism of a DNA polymerase sliding clamp loader.Elife. 2022 Feb 18;11:e74175. doi: 10.7554/eLife.74175. Elife. 2022. PMID: 35179493 Free PMC article.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Kelch B. A., The lord of the rings: Structure and mechanism of the sliding clamp loader. Biopolymers 105, 532–546 (2016). - PubMed
-
- Moldovan G.-L., Pfander B., Jentsch S., PCNA, the maestro of the replication fork. Cell 129, 665–679 (2007). - PubMed
-
- Erzberger J. P., Berger J. M., Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006). - PubMed
-
- Davey M. J., Jeruzalmi D., Kuriyan J., O’Donnell M., Motors and switches: AAA+ machines within the replisome. Nat Rev Mol Cell Biol 3, 826–835 (2002). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
