This is a preprint.
Fusion of Complement Fragment C3d Enhances Germinal Center Responses to HIV-1 Envelope Glycoproteins
- PMID: 40631158
- PMCID: PMC12236797
- DOI: 10.1101/2025.06.28.661730
Fusion of Complement Fragment C3d Enhances Germinal Center Responses to HIV-1 Envelope Glycoproteins
Abstract
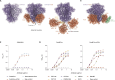
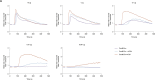
Eliciting sustained germinal center (GC) responses is critical for the development of an effective HIV-1 vaccine, yet HIV-1 envelope glycoprotein (Env) immunogens often fail to elicit GC responses required for the maturation of cognate B cells that secrete broadly neutralizing antibodies (bNAbs). Effective antigen recognition is important for initial B cell priming, activation, and GC engagement. Since complement opsonization contributes to antigen recognition, we investigated whether C3d fusion could enhance the GC response of the stabilized HIV-1 Env immunogen based on a consensus sequence (ConM Env). Our results demonstrate that ConM Env-C3d induced potent HIV-specific B cell activation in vitro compared to ConM Env alone. We also observed that the C3d fusion enhanced antigen presentation by human tonsil-derived follicular dendritic cells (FDCs) to HIV-specific B cell lines. Moreover, mouse immunization studies combining ConM Env-C3d with the AddaS03 adjuvant revealed significantly enhanced early GC formation and prolonged antigen display and retention on FDCs for up to 56 days, highlighting improved antigen persistence within GCs. These immunological enhancements, including a more focused early antibody response, correlated with improved virus neutralization. Additionally, we observed sex-based differences in immune responses, with female mice showing stronger antibody responses and enhanced antigen retention compared to males. These findings suggest that C3d fusion can enhance GC engagement and improve the immunogenicity of HIV-1 vaccines.
Keywords: B cell activation; FDCs; HIV-1 Env; antigen retention; complement C3d; germinal center response; neutralizing antibodies.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
