This is a preprint.
Robust Production of Parvalbumin Interneurons and Fast-Spiking Neurons from Human Medial Ganglionic Eminence Organoids
- PMID: 40631166
- PMCID: PMC12236662
- DOI: 10.1101/2025.07.01.662594
Robust Production of Parvalbumin Interneurons and Fast-Spiking Neurons from Human Medial Ganglionic Eminence Organoids
Abstract
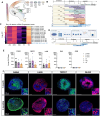
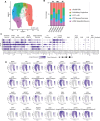
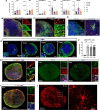
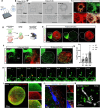
The medial ganglionic eminence (MGE) gives rise to parvalbumin (PV)- and somatostatin (SST)-expressing cortical interneurons essential for regulating cortical excitability. Although PV interneurons are linked to various neurodevelopmental and neurodegenerative disorders, reliably generating them from human pluripotent stem cells (hPSCs) has been extremely challenging. We present a robust, reproducible protocol for generating single-rosette MGE organoids (MGEOs) from hPSCs. Transcriptomic analyses reveal that MGEOs exhibit MGE regional identity and faithfully model the developing human fetal MGE. As MGEOs mature, they generate abundant PV-expressing cortical interneurons, including putative basket and axoaxonic cells, at a scale not previously achieved in vitro. When fused with hPSC-derived cortical organoids, these interneurons rapidly migrate into cortical regions, integrate into excitatory networks, and contribute to complex electrophysiological patterns and the emergence of large numbers of fast-spiking neurons. MGEOs thus offer a powerful in vitro approach for probing human MGE-lineage cortical and subcortical GABAergic neuron development, modeling various neuropsychiatric disorders, and advancing cell-based therapies for neurodevelopmental and neurodegenerative disorders.
Keywords: GABAergic; axoaxonic cells; basket cells; brain organoid; forebrain development; multielectrode array; neuronal migration; pluripotent stem cells.
Conflict of interest statement
Declaration of interests The authors have no financial or other competing interests to declare.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
