This is a preprint.
SARS-CoV-2 infection disrupts syncytial and endothelial integrity and alters PLGF levels in the placenta
- PMID: 40631276
- PMCID: PMC12236811
- DOI: 10.1101/2025.06.27.661568
SARS-CoV-2 infection disrupts syncytial and endothelial integrity and alters PLGF levels in the placenta
Update in
-
SARS-CoV-2 infection disrupts syncytial and endothelial integrity and alters PlGF levels in the placenta.Placenta. 2025 Nov;171:34-44. doi: 10.1016/j.placenta.2025.09.009. Epub 2025 Sep 14. Placenta. 2025. PMID: 40966962
Abstract
Introduction: SARS-CoV-2 infection during pregnancy has been associated with an increased risk for several pregnancy-related disorders, particularly preeclampsia (PE). However, there are limited studies determining the impact of SARS-CoV-2 on placental physiology and function.
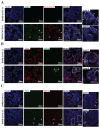
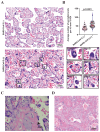
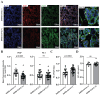
Methods: Placental samples were acquired from two large prospective cohorts: STOP-COVID19 and REBRACO studies. Placental villous tissues (VTs) were collected from pregnant women who tested positive for SARS-CoV-2 without PE during pregnancy. Immunohistochemistry and immunofluorescence were used to assess pathological features known to be altered in PE, including 1) syncytial knot formation; 2) alterations in renin-angiotensin system components; 3) and endothelial integrity. Maternal serum was collected to examine AT1 autoantibodies levels using an immunoassay.
Results: SARS-CoV-2 viral proteins spike, nucleocapsid, and ORF3a were observed in the syncytiotrophoblast layer and stroma of placental VT. SARS-CoV-2-infected placentas exhibited increased numbers of syncytial knots, which were positive for Flt-1 and SARS-CoV-2 viral proteins. In addition, the presence of placental infarctions and excessive fibrin deposits was also observed in infected placentas. Infection was associated with decreased placental expression of PlGF and an increase in the placental Flt-1/PlGF expression ratio, mostly driven by PlGF. No significant changes in maternal serum AT1AA levels were observed. Finally, SARS-CoV-2-infected placentas exhibited a significant decrease in vimentin expression.
Discussion: SARS-CoV-2 infection negatively impacts placental integrity in the form of increased syncytial knots, dysregulated RAS components, and endothelial damage. Since all these features are similarly disrupted in PE, this could be a mechanism through which SARS-CoV-2 infection during pregnancy increases the risk of a PE-like syndrome.
Keywords: COVID-19; Preeclampsia; Renin Angiotensin system; Syncytial knots, placenta.
Conflict of interest statement
Declaration of interest IUM serves on the scientific advisory board of Seed Health. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Sathiya R., Rajendran J., Journal S.S.-M.M., undefined 2022, COVID-19 and Preeclampsia: Overlapping Features in Pregnancy, Ncbi.Nlm.Nih.Gov (n.d.). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8798587/ (accessed December 6, 2022). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
