This is a preprint.
Single-cell RNA sequencing in Hirschsprung's disease tissues reveals lack of neuronal differentiation in the aganglionic colon segment
- PMID: 40631286
- PMCID: PMC12236654
- DOI: 10.1101/2025.07.01.662516
Single-cell RNA sequencing in Hirschsprung's disease tissues reveals lack of neuronal differentiation in the aganglionic colon segment
Abstract
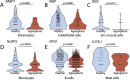
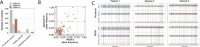
The enteric nervous system (ENS) is a complex network of neurons and glial cells. Hirschsprung's disease (HSCR) is a congenital condition characterized by the absence of ganglion cells in the distal colon, leading to functional bowel obstruction. In this study, we used single-cell RNA sequencing (scRNA-seq) and whole genome sequencing (WGS) to analyze healthy and aganglionic colon segments from HSCR patients. Using scRNA-seq, we identified 13 major cell types in patient samples and observed that neural progenitor cells were present in both healthy and aganglionic colon regions, while mature neurons were absent from aganglionic colon. In these progenitor cells, critical differentiation pathway genes displayed reduced expression in the aganglionic colon, suggesting a disruption in their transition to mature neuronal cell types. Furthermore, transcriptomic analysis revealed significant alterations in gene expression across several stromal cell types. These transcriptomic shifts, particularly in mast cells, support the hypothesis that altered gene expression in the microenvironment of neural progenitor cells contributes to impaired differentiation. Our findings support the hypothesis that neural precursors in HSCR are capable of migration, but they are defective in their differentiation to mature cell types. Our analysis provides insights into potential therapeutic targets to stimulate neurogenesis in the aganglionic colon.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Furness JB, et al. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control [preprint]. Advances in Experimental Medicine and Biology. 2014;39–71. - PubMed
-
- Haricharan RN, Georgeson KE. Hirschsprung disease. Semin Pediatr Surg. 2008;17(4):266–275. - PubMed
-
- Skinner MA. Hirschsprung’s disease. Curr Probl Surg. 1996;33(5):389–460. - PubMed
-
- Thakkar HS, et al. Functional outcomes in Hirschsprung disease: A single institution’s 12-year experience. J Pediatr Surg. 2017;52(2):277–280. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
