This is a preprint.
Response of human iPSC-cardiomyocytes to adrenergic drugs assessed by high-throughput pericellular oxygen measurements
- PMID: 40631304
- PMCID: PMC12236770
- DOI: 10.1101/2025.06.27.662066
Response of human iPSC-cardiomyocytes to adrenergic drugs assessed by high-throughput pericellular oxygen measurements
Abstract
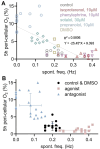
Rate-modulating drugs, such as adrenergic agonists and antagonists, are widely used in the treatment of cardiovascular conditions. Preclinical assessment of new rate and metabolism modulators can be augmented through the development of high-throughput (HT) methods that allow chronic measurements. Such approaches are best coupled with human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) - a scalable experimental model of the human heart. Here, we evaluate the utility of long-term optical (label-free) measurements of pericellular oxygen in a HT format (96-well plates) for the assessment of the effectiveness of adrenergic drugs in hiPSC-CMs. Quantitative metrics were derived from these long-term measurements, e.g. steady-state pericellular oxygen and time to reach 5%, and we sought correlation to measurements performed in the same samples using all-optical electrophysiology. Adrenergic agonists significantly increased oxygen consumption rate, and this was best seen in the kinetics of initial depletion of pericellular oxygen, i.e. time to reach 5%. Adrenergic antagonists decreased oxygen consumption rate and their action was best quantified using steady-state values for pericellular oxygen after at least 5 hours. Drug type identification based on oxygen consumption rate correlated well with the acute measurements of spontaneous rate in the same samples. Furthermore, we showed that direct rate modulation with chronic optogenetic pacing detectably sped up the oxygen consumption rate and optogenetic transformation did not interfere with classification of adrenergic drugs. We conclude that HT label-free optical oxygen measurements may be a valuable approach for long-term non-invasive monitoring of the action of rate- and metabolism-modulating drugs in preclinical studies.
Keywords: adrenergic agonists; all-optical electrophysiology; beta blockers; high-throughput plates; human iPSC-CMs; optical oxygen sensors; pericellular oxygen.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures





Similar articles
-
Pericellular oxygen dynamics in human cardiac fibroblasts and iPSC-cardiomyocytes in high-throughput plates: insights from experiments and modeling.J Mol Cell Cardiol Plus. 2025 Jun 11;13:100464. doi: 10.1016/j.jmccpl.2025.100464. eCollection 2025 Sep. J Mol Cell Cardiol Plus. 2025. PMID: 40600000 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Ahlquist R. P. (1948). “A STUDY OF THE ADRENOTROPIC RECEPTORS.” American Journal of Physiology-Legacy Content 153(3): 586–600. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
