This is a preprint.
High-fat diet ablates an insulin-responsive pool of GLUT4 glucose transporters in skeletal muscle
- PMID: 40631311
- PMCID: PMC12236751
- DOI: 10.1101/2025.06.29.662135
High-fat diet ablates an insulin-responsive pool of GLUT4 glucose transporters in skeletal muscle
Abstract
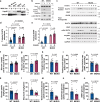
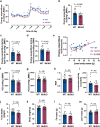
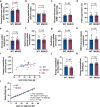
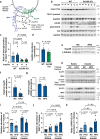
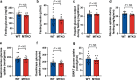
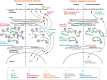
To stimulate glucose uptake in muscle, insulin mobilizes GLUT4 glucose transporters to the cell surface. During fasting, GLUT4 and the transmembrane aminopeptidase IRAP are trapped in intracellular, insulin-responsive vesicles bound by TUG, AS160, and Usp25m proteins. Here we show that Usp25m, a protease, is required for the bulk of insulin-stimulated TUG cleavage and consequent vesicle mobilization and glucose uptake. Efficient TUG cleavage also requires AS160. In mice with diet-induced insulin resistance, Usp25m abundance is reduced, IRAP is mislocalized during fasting, and TUG cleavage is impaired; effects of Usp25m and TUG deletion to alter insulin-stimulated and fasting glucose uptake, respectively, are ablated. We conclude that skeletal muscle insulin resistance results in part from altered membrane trafficking of GLUT4 and IRAP during fasting. This alteration depletes the pool of insulin-responsive vesicles marked by TUG and Usp25m. Mistargeting of GLUT4 and IRAP may contribute to distinct aspects of the metabolic syndrome in humans.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
