Sunitinib as Second-Line Treatment in Advanced Intrahepatic Cholangiocarcinoma: Results From the SUN-CK GERCOR Phase II Trial
- PMID: 40631456
- PMCID: PMC12239060
- DOI: 10.1111/liv.70196
Sunitinib as Second-Line Treatment in Advanced Intrahepatic Cholangiocarcinoma: Results From the SUN-CK GERCOR Phase II Trial
Abstract
Background & aims: Angiogenesis is critical in intrahepatic cholangiocarcinoma (ICC), a highly lethal cancer with limited treatment options. Sunitinib, a multi-receptor tyrosine kinase inhibitor, has strong antiangiogenic and antitumor effects. We aimed to evaluate the efficacy and tolerability of sunitinib as a second-line treatment in chemotherapy-pretreated patients with advanced ICC.
Methods: This open-label, single-arm, phase II trial was conducted across five French centres. Eligible patients were aged ≥ 18, had advanced ICC not suitable for curative surgery and had been pre-treated with gemcitabine and/or platinum. Patients received oral sunitinib at 37.5 mg daily. The primary endpoint was overall survival (OS), with secondary endpoints including progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR) and safety. Tumour response was evaluated using RECIST v1.1, with exploratory analysis using Choi criteria. VEGF-A, VEGF-C and plasma sunitinib levels were measured.
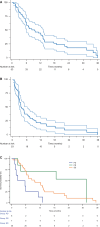
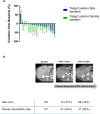
Results: Fifty-three patients were included. Median OS was 9.6 months (95% CI, 6.1-13.1), median PFS was 3.7 months (95% CI, 3.1-6.4), the ORR was 14% and the DCR was 84%. Grade 3-4 cytopenias occurred in 25% of patients, hypertension in 21% and fatigue in 19%. Higher baseline levels of VEGF-A and VEGF-C correlated with longer OS. Patients with dose reductions maintained adequate drug exposure. Choi criteria, applied to 24 patients, predicted response duration better than RECIST v1.1. Baseline tumour density, assessed in 14 patients, showed a potential association with treatment responses.
Conclusions: Sunitinib demonstrated efficacy and manageable toxicity as a second-line treatment in patients with advanced ICC previously treated with chemotherapy, suggesting it may represent a viable therapeutic option for this population.
Trial registration: ClinicalTrials.gov NCT01718327.
Keywords: Choi criteria; VEGF; angiogenesis inhibitors; biliary tract cancer; tumour density.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
Mohamed Bouattour reports consulting fees from AstraZeneca, Bayer, Bristol‐Myers Squibb, Eisai, Ipsen, MSD, Roche and Sirtex Medical; speakers bureau from Bayer, Eisai and Roche and support for travel/attending meetings from AstraZeneca, Bayer and Sirtex Medical. Clément Dumont reports occasional engagements for Astellas, BMS, Janssen, MSD and Pfizer. David Malka has received consulting fees/honoraria from AbbVie, Amgen, AstraZeneca, Bayer, BMS, Foundation Medicine, Incyte, Leo Pharma, Merck Serono, MSD, Pierre Fabre Oncologie, Roche, Sanofi, Servier, Taiho and Viatris and travel expenses from Amgen, Bayer, BMS, Merck Serono, MSD, Pierre Fabre Oncologie, Roche, Sanofi, Servier and Viatris. Annemilaï Tijeras‐Raballand Employment: AAREC Filia Research. Travel, Accommodations, Expenses: Merck KGaA. Armand De Gramont Employment: AAREC Filia Research. Consulting or Advisory Role: PharmaEngine Travel, Accommodations, Expenses: Celgene. Maxime Ronot Honoraria from Alexion Pharmaceuticals, Canon‐Toshiba, GE Healthcare, Guerbet, Ipsen, Servier, Sirtex, Terumo; support for meetings from Baxter. Cindy Neuzillet has received consulting fees/honoraria from Amgen, Astellas, AstraZeneca, AAA, Baxter, Bristol‐Myers Squibb, Boehringer Ingelheim, Fresenius Kabi, Incyte Biosciences, Merck, MSD, Mundipharma, Nestlé Health Science, Novartis, Nutricia, OSE Immunotherapeutics, Pierre Fabre, Roche, Sanofi, Servier, Viatris; research funding (to institution) from AstraZeneca, Bristol‐Myers Squibb, Fresenius Kabi, Nutricia, OSE Immunotherapeutics, Roche, Servier, Viatris. Benoist Chibaudel has had a consulting or advisory role with Bayer, Lilly, Roche and Sanofi and has received travel, accommodations and expenses from Amgen, Lilly, Merck, Roche and Sanofi. Eric Raymond reports serving as a consultant for SCOR, Genoscience Pharma, StromaCare and Onward therapeutic and is a shareholder for SCOR, Genoscience Pharma, StromaCare and Axoltis. Sandrine Faivre is a consultant for and has received clinical trials grants from, Bristol‐Myers Squibb, Bayer Pharma, MSD, Novartis and Pfizer.
Figures
References
-
- Vogel A., Bridgewater J., Edeline J., et al., “Biliary Tract Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow‐Up,” Annals of Oncology 34 (2023): 127–140. - PubMed
-
- Bertuccio P., Malvezzi M., Carioli G., et al., “Global Trends in Mortality From Intrahepatic and Extrahepatic Cholangiocarcinoma,” Journal of Hepatology 71 (2019): 104–114. - PubMed
-
- Delaye M., Boilève A., Henriques J., et al., “Real‐Life Data on Biliary Tract Cancers in France: The Nested Amber Study From the French ACABi GERCOR PRONOBIL Retro‐Prospective, Observational Cohort,” Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 57 (2025): 111–117, 10.1016/j.dld.2024.06.032. - DOI - PubMed
-
- Primrose J. N., Fox R. P., Palmer D. H., et al., “Capecitabine Compared With Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study,” Lancet Oncology 20 (2019): 663–673. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

