Cardiac amyloidosis detection from a single echocardiographic video clip: a novel artificial intelligence-based screening tool
- PMID: 40631729
- PMCID: PMC12539910
- DOI: 10.1093/eurheartj/ehaf387
Cardiac amyloidosis detection from a single echocardiographic video clip: a novel artificial intelligence-based screening tool
Abstract
Background and aims: Accurate differentiation of cardiac amyloidosis (CA) from phenotypic mimics remains challenging using current clinical and echocardiographic techniques. The accuracy of a novel artificial intelligence (AI) screening algorithm for echocardiography-based CA detection was assessed.
Methods: Utilizing a multisite, multiethnic dataset (n = 2612, 52% CA), a convolutional neural network was trained to differentiate CA from phenotypic controls using transthoracic apical four-chamber video clips. External validation was conducted globally across 18 sites including 597 CA cases and 2122 controls. Classification accuracy was assessed on the entire external validation dataset, and subgroup analyses were performed both on technetium pyrophosphate scintigraphy referrals, and individuals matched for age, sex, and wall thickness. Model accuracy was also compared with the transthyretin CA score and the increased wall thickness score within a subset of older heart failure with preserved ejection fraction patients with increased wall thickness.
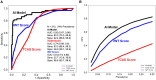
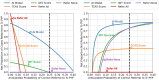
Results: Cardiac amyloidosis patients and controls displayed similar age, sex, race, and comorbidities. After the removal of uncertain AI predictions (13%), model discrimination and classification were excellent for the entire external validation dataset [area under the receiver operating characteristic curve (AUROC) 0.93, sensitivity 85%, specificity 93%], irrespective of CA subtype (sensitivity: light-chain = 84%, wild-type transthyretin = 85%, and hereditary transthyretin = 86%). Performance was maintained in subgroup analysis in patients clinically referred for technetium pyrophosphate scintigraphy imaging (AUROC 0.86, sensitivity 77%, specificity 86%) and matched patients (AUROC 0.92, sensitivity 84%, specificity 91%). The AI model (AUROC 0.93) also outperformed transthyretin CA score (AUROC 0.73) and increased wall thickness (AUROC 0.80) scores.
Conclusions: This AI screening model-using only an apical four-chamber view-effectively differentiated CA from other causes of increased left ventricular wall thickness.
Keywords: Amyloid; Artificial intelligence; Cardiomyopathy; Echocardiography; Heart failure.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


References
-
- Writing C, Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol 2023;81:1076–126. 10.1016/j.jacc.2022.11.022 - DOI - PubMed
-
- Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. Circ Cardiovasc Imaging 2021;14:e000029. 10.1161/HCI.0000000000000029 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

