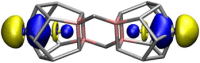
Molecular-Metallic Binding Characteristics of the Intermetalloid f-/p-Block Cluster [(La@In2Bi11)2Bi2]6
- PMID: 40631730
- PMCID: PMC12416455
- DOI: 10.1002/anie.202512019
Molecular-Metallic Binding Characteristics of the Intermetalloid f-/p-Block Cluster [(La@In2Bi11)2Bi2]6
Abstract
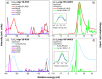
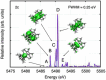
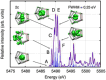
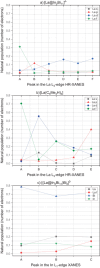
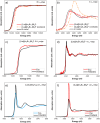
Main goals of contemporary research in chemistry are to create new materials with unique properties and to understand the chemical bonding in them, especially between metal atoms in larger structures. The isolation of a single lanthanide atom in a In/Bi cage offers a non-standard bonding situation, which deserves thorough exploration. In this study, the bonding behavior of La, In, and Bi atoms in the ternary cluster [(La@In2Bi11)2Bi2]6- and the complex [La(C5Me4H)3] used for its synthesis are characterized and compared by applying high energy resolution X-ray spectroscopy and computations. A clearly detectable covalent La(5d)─Bi(6p) interaction, induced in a highly electron-rich environment, is illustrated. The electronic structure of the La atom can be described as having the character of an ion being trapped and bonded in a heterometallic Bi/In cage. The advanced X-ray spectroscopic experimental tools applied here enable comparative studies of binding properties, focusing on different metals within the intermetalloid cluster. These tools can be employed iteratively to support the development of synthetic strategies that aim at tuning bond characteristics at the boundary of covalent and metallic bonding, thereby advancing the chemical and physical properties of novel multinary cluster compounds. The results were corroborated by GW and Bethe-Salpeter-equation (GW-BSE) calculations.
Keywords: Covalency of Ln‐based bonds; GW‐BSE calculations; Intermetallic clusters; La HR‐XANES; La VB‐RIXS.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fässler T. F., Hoffmann S. D., Angew. Chem. Int. Ed. 2004, 43, 6242–6247. - PubMed
-
- Fässler T. F., Zintl Ions: Principles and Recent Developments, (Ed.: T.F. Fässler), Springer, New York: 2011, pp. 91–131.
-
- Scharfe S., Kraus F., Stegmaier S., Schier A., Fässler T. F., Angew. Chem. Int. Ed. 2011, 50, 3630–3670. - PubMed
-
- Weinert B., Dehnen S., Clusters – Contemporary Insight in Structure and Bonding, (Ed.: Dehnen S.), Springer, New York: 2017, pp. 99–134.
-
- Wilson R. J., Weinert B., Dehnen S., Dalton Trans. 2018, 47, 14861–14869. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

