First-in-human phase I open-label study of the LAG-3 antagonist antibody INCAGN02385 in patients with select advanced or metastatic solid tumors
- PMID: 40631774
- PMCID: PMC12238943
- DOI: 10.1093/oncolo/oyaf136
First-in-human phase I open-label study of the LAG-3 antagonist antibody INCAGN02385 in patients with select advanced or metastatic solid tumors
Abstract
Background: Immune checkpoint receptor lymphocyte-activation gene 3 (LAG-3) is an activation marker for CD4+ and CD8+ T cells. Prolonged LAG-3 expression downregulates T-cell activation; therefore, LAG-3 blockade may restore antitumor immune response. INCAGN02385 is a humanized monoclonal LAG-3-targeting antibody. This first-in-human phase I study evaluated INCAGN02385 for advanced/metastatic solid tumors.
Materials and methods: In this dose escalation study, patients with select immunogenic advanced or metastatic solid tumors received a single INCAGN02385 infusion (25 mg to 750 mg) every 2 weeks (Q2W). Objectives included evaluation of safety/tolerability, maximum tolerated dose (MTD) (primary), pharmacokinetics (PK), antitumor activity (secondary).
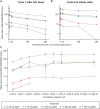
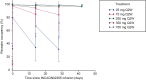
Results: Twenty-two patients were enrolled and treated. Sixty-four percent had received ≥ 3 lines of systemic therapy. Sixty-eight percent had received prior immune checkpoint inhibitor (ICI) therapy; anti-programmed death protein-1/anti-programmed death ligand-1, 68%, anti-cytotoxic T-lymphocyte-associated protein-4 therapy, 18%. No dose-limiting toxicities occurred, and an MTD was not reached. Sixteen patients (73%) experienced treatment-related adverse events (TRAEs), most frequently fatigue (n = 7). Except for one grade 3 lymphopenia TRAE, all were grade 1/2 severity. Two patients experienced sponsor-assessed immune-related AEs (pneumonitis, peripheral sensory neuropathy [n = 1] patient each). INCAGN02385 PK parameters were dose proportional across all doses evaluated. Six patients achieved stable disease lasting ≥ 56 days (range, 57-413 days).
Conclusions: INCAGN02385 exhibited linear PK and preliminary evidence of disease control in this heavily pretreated population, consistent with other LAG-3-targeting monotherapies. A 350-mg Q2W dose was selected for phase II studies that will focus on combinations of INCAGN02385 with other ICIs.
Keywords: carcinoma; clinical trial phase I; immune checkpoint inhibitors; immunotherapy; metastases; monoclonal antibodies.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
J.D.P. reports being a Board of Directors member, with ownership interest and intellectual property, for BioCytics and Carolina BioOncology Institute; founder and owner of BioCytics; founder and owner of a phase 1 cancer research clinic; developing intellectual property for cellular therapies and developing immune cellular therapy as founder/owner of BioCytics; consultant for Aavocyte and Boxer Capital; principal investigator for, without financial interest in, AbbVie, Adagene, Alkermes, Apollo, Apros Therapeutics, Arcus BioSciences, Astellas, AstraZeneca/MedImmune, Atreca, BioNTech, BJ BioScience, Bristol Myers Squibb, Calico Life Sciences, Conjupro Biotherapeutics, Corbus, Cullinan Therapeutics, CytoMx, EMD Serono, Fate Therapeutics, FBD Biologics, FLX Bio, Genentech/Roche, Glenmark, I-MAB Biopharma, Immune-Onc Therapeutics, Incyte, Jounce Therapeutics, MacroGenics, MBrace, Molecular Templates, Multitude, NextCure, Nuvation Bio, Repertoire Immune Medicines, Revolution Medicines, Sairopa, Seattle Genetics, Sequenom, SK Life Science, Tempest Therapeutics, Top Alliance Biosciences, Trethera, Xilio Therapeutics, and Zenshine Pharmaceuticals; institutional funding, with no financial interest, from Merck, MT Group, Precision for Medicine, Replimune, STEMCELL Technologies, and Xilis; principal investigator for, with financial interest in, Allarity, Aptevo, Aulos, CUE BioPharma, GI Innovation, Harbour BioMed, IconOVir Bio, IGM BioSciences, Medikine, Moderna TX, PEEL Therapeutics, Phanes Therapeutics, Pieris Pharmaceuticals, Qurgen, RiboScience, Simcere, and SK Life Science; personal and institutional funding from Aavocyte, AbbVie, Adagene, Alkerme, Allarity, Apollo, Apros, Aptevo, Arcus BioSciences, Astellas, AstraZeneca/MedImmune, Atreca, Aulos, BioNTech, BJ BioScience, Bristol Myers Squibb, Calico Life Sciences, Conjupro Biotherapeutics, Corbus, CUE BioPharma, Cullinan, CytoMx, EMD Serono, Fate Therapeutics, FBD Biologics, FLX Bio, Genentech/Roche, GI Innovation, Glenmark, Harbour BioMed, I-MAB Pharma, IconOVIR Bio, IGN BioSciences, Immune-Onc, Incyte, Jounce Therapeutics, MacroGenics, MBrace, Medikine, Moderna TX, Molecular Templates, Multitude, NextCure, Nuvation, PEEL Therapeutics, Phanes Therapeutics, Pieris Pharmaceuticals, PIOMA, Qurgen, Repertoire Immune Medicines, Revolution Medicines, RiboScience, Sairopa, Seattle Genetics, Sequenom, Simcere, SK Life Science, Tempest Therapeutics, Top Alliance BioScience, Trethera, Wugen, Xilio Therapeutics, and Zenshine Pharma; funding, with a financial interest, from PIOMA and Wugen; Wugen is sponsor of contract laboratory translational research; writing engagement, personal, and other, with financial interest, from Aavocyte; Aavocyte and AavoBioCytics are jointly developing cellular therapies with BioCytics Human Applications Lab for point-of-care manufacturing; personal, from Boxer Capital; clinical trial funding from Apollo, Astellas, BioNTech, Corbus, CytoMx, FBD Biologics, Glenmark, MBrace, Multitude, Revolution Medicines, Sairopa, and SK Life Science; and funding for contract laboratory services from Nuvation. M.E.G. reports stock and other ownership interests from COTA Healthcare; speakers’ bureaus for Bristol Myers Squibb, Eli Lilly & Company, and Merck; research funding from Acerta Pharma, Adlai Nortye, Arcus Biosciences, Array BioPharma, Bayer, Bellicum Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Checkpoint Therapeutics, Compass Therapeutics, Constellation Pharmaceuticals, Cullinan Oncology, Cyteir Therapeutics, Daiichi Sankyo, Eisai, EMD Serono, Erasca, Fate Therapeutics, Georgetown University, GlaxoSmithKline, GSB Pharma, Hackensack Meridian Health, Imugene, Incyte Corporation, Infinity Pharmaceuticals, iTeos Therapeutics, Janssen, Johnson & Johnson, KSQ Therapeutics, MedImmune, Memorial Sloan Kettering Cancer Center, Merck, Millennium Pharmaceuticals, Mirati Therapeutics, Moderna, NextCure, Nimbus Therapeutics, Pfizer, Pharmacyclics, Rapa Therapeutics, Regeneron, Roche/Genentech, Sanofi, Seagen, Silenseed, Synlogic Therapeutics, TESARO, Turning Point Therapeutics, Vedanta Biosciences, VelosBio, Verastem Oncology, and Vincerx Pharma; and travel, accommodations, and expenses from Guardant Health. A.S.B. reports speakers’ bureaus for AstraZeneca, Bristol Myers Squibb, Genentech, Merati, Merck, and Regeneron; steering committee for Janssen (Johnson & Johnson) (2023, 2024); and consultant for AbbVie (2024) and Pfizer (2023). P.E.H., Z.D., L.C., X.C., and J.E.J., report employment by and stock ownership in Incyte Corporation. N.B. reports employment by and stock ownership in Incyte Biosciences International Sàrl. O.H. reports honoraria from Bristol Myers Squibb, Immunocore, Novartis, Pfizer, and Regeneron; has served as a consultant or advisor to Alkermes, Amgen, Bactonix, BeiGene, BioAtla, Bristol Myers Squibb, Eisai, Georgiamune, GigaGen, GlaxoSmithKline, Grit Biotechnology, Idera, Immunocore, Incyte Corporation, Instil Bio, Iovance Biotherapeutics, Janssen, KSQ Therapeutics, Merck, Moderna, Novartis, Obsidian, Pfizer, Regeneron, Roche/Genentech, Sanofi, Seattle Genetics, Tempus, Vial Health, and Zelluna Immunotherapy; has participated in speakers’ bureaus for Bristol Myers Squibb, Immunocore, Novartis, Pfizer, and Regeneron; has received institutional research funding from Arcus Biosciences, Aduro, Akeso, Amgen, BioAtla, Bristol Myers Squibb, CytomX Therapeutics, Exelixis, GlaxoSmithKline, Idera, Immunocore, Incyte Corporation, Iovance Biotherapeutics, Merck, Merck Serono, Moderna, NextCure, Novartis, Pfizer, Regeneron, Roche/Genentech, Seattle Genetics, Torque Pharmaceuticals, and Zelluna Immunotherapy.
Figures

References
-
- Sun J-Y, Zhang D, Wu S, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res. 2020;8:35. https://doi.org/ 10.1186/s40364-020-00212-5 - DOI - PMC - PubMed
-
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175. https://doi.org/ 10.1200/JCO.2009.26.7609 - DOI - PMC - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. https://doi.org/ 10.1056/NEJMoa1200690 - DOI - PMC - PubMed
-
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti–PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286-4293. https://doi.org/ 10.1158/1078-0432.CCR-14-2607 - DOI - PubMed
-
- Zhao B, Zhao H, Zhao J.. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. 2020;12:1758835920937612. https://doi.org/ 10.1177/1758835920937612 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

