Moving the needle on immune checkpoint inhibitors with novel targets: are we being TIMid or LAGging behind?
- PMID: 40631775
- PMCID: PMC12238944
- DOI: 10.1093/oncolo/oyaf145
Moving the needle on immune checkpoint inhibitors with novel targets: are we being TIMid or LAGging behind?
Conflict of interest statement
PLB: Consultant/Advisory role for: Zymeworks, Lilly, Seattle Genetics, Merck, Amgen, Gilead, Jannsen, Repare, Daiichi Sankyo. Grant/Research support from (Clinical Trials): Amgen, Astra Zeneca, Bayer, Bicara, Boehringer Ingelheim, BristolMyersSquibb, Daiichi Sankyo, Genentech/Roche, Gilead, GlaxoSmithKline, Lilly/Loxo, Medicenna, Merck, Nektar, Novartis, PTC Therapeutics, Sanofi, SeaGen, Servier, SignalChem Life Sciences, Takeda, Zymeworks
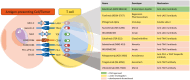
Figures
References
-
- He X, Xu C.. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660-669. https://doi.org/ 10.1038/s41422-020-0343-4 - DOI - PMC - PubMed
-
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. https://doi.org/ 10.1016/S1470-2045(15)70076-8 - DOI - PubMed
-
- Walsh RJ, Sundar R, Lim JSJ.. Immune checkpoint inhibitor combinations-current and emerging strategies. Br J Cancer. 2023;128:1415-1417. https://doi.org/ 10.1038/s41416-023-02181-6 - DOI - PMC - PubMed
-
- Maruhashi T, Sugiura D, Okazaki I-mi, Okazaki T.. LAG-3: from molecular functions to clinical applications. J ImmunoTher Cancer. 2020;8:e001014. https://doi.org/ 10.1136/jitc-2020-001014 - DOI - PMC - PubMed
-
- Long L, Zhang X, Chen F, et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9:176-189. https://doi.org/ 10.18632/genesandcancer.180 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

