Talquetamab improves patient-reported symptoms and health-related quality of life in relapsed or refractory multiple myeloma: Results from the phase 1/2 MonumenTAL-1 study
- PMID: 40631904
- PMCID: PMC12239699
- DOI: 10.1002/cncr.35927
Talquetamab improves patient-reported symptoms and health-related quality of life in relapsed or refractory multiple myeloma: Results from the phase 1/2 MonumenTAL-1 study
Abstract
Background: Talquetamab is the first approved G protein-coupled receptor family C group 5 member D-targeting bispecific antibody for the treatment of triple-class exposed relapsed/refractory multiple myeloma (RRMM) on the basis of results from the phase 1/2 MonumenTAL-1 study (ClinicalTrials.gov identifiers NCT03399799 and NCT04634552). This study describes patient-reported outcomes (PROs) among patients who received talquetamab in MonumenTAL-1.
Methods: PROs were assessed in phase 2 of MonumenTAL-1. Patients had RRMM with ≥3 prior lines of therapy (proteasome inhibitors, immunomodulatory drugs, and anti-CD38 antibodies). Patients included in these analyses received talquetamab 0.8 mg/kg every other week. PRO assessments included European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), EuroQol 5-Dimension 5-Level Visual Analogue Scale (EQ-5D-5L VAS), and Patient Global Impression of Severity (PGIS).
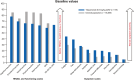
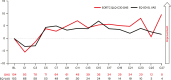
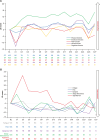
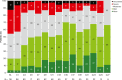
Results: As of January 29, 2024, PRO data were available for 118 patients who received ≥1 dose of talquetamab. Compliance for completion was >95% of patients at baseline and >80% at most posttreatment visits. After an initial worsening in most PROs from baseline through cycle 1, patients reported improvements with continued treatment. At cycle 21, least-squares mean change from baseline showed improvements in global health status (4.9 points) and emotional functioning (12.5 points), along with reductions in pain (11.4 points) and fatigue (4.0 points). The proportion of patients with clinically meaningful improvements or no change from baseline increased throughout treatment. EQ-5D-5L VAS and PGIS scores demonstrated improvement in health-related quality of life.
Conclusions: These PRO results complement the efficacy and safety profile of talquetamab, which highlights talquetamab as a viable treatment option for patients with triple-class exposed RRMM.
Keywords: bispecific antibody; health‐related quality of life; multiple myeloma; patient‐reported outcome measures.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Carolina Schinke has received honoraria from Johnson & Johnson and Pfizer, and has participated in advisory board meetings for Arcellx, Johnson & Johnson, and Pfizer. Cyrille Touzeau has received honoraria from Bristol‐Myers Squibb (BMS) and Janssen Biotech, and serves as a member on the board of directors or advisory committee for BMS. Albert Oriol has received honoraria from Amgen, BMS, GlaxoSmithKline (GSK), Janssen Biotech, Johnson & Johnson, Menarini, Oncopeptides, Pfizer, and Sanofi, and serves as a member on the board of directors or advisory committee for BMS, GSK, Johnson & Johnson, Menarini, Oncopeptides, Pfizer, and Sanofi. María‐Victoria Mateos has received honoraria from AbbVie, Amgen, BMS/Celgene, GSK, Johnson & Johnson, Kite Pharma, Oncopeptides, Pfizer, Regeneron, Roche, Sanofi, Stemline, and Takeda; is a member of a board of directors or advisory committee for AbbVie, BMS/Celgene, GSK, Janssen Global Services, Johnson & Johnson, Oncopeptides, Pfizer, Sanofi, Stemline, and Takeda; and is a speakers’ bureau member for Johnson & Johnson. Leo Rasche has received honoraria from BMS, GSK, Johnson & Johnson, Pfizer, Roche, and Sanofi; is a member of a board of directors or advisory committee for Amgen, BMS, Johnson & Johnson, Pfizer, and Sanofi; is a consultant for Amgen, BMS, GSK, Johnson & Johnson, Pfizer, and Sanofi; has received travel support from BeiGene and Johnson & Johnson; and has received research funding from BMS, GSK, Sanofi, and SkylineDx. Xiang Qin, Kelly Kato, Sacheeta Bathija, Eva G. Katz, Katharine S. Gries, Michela Campagna, Tara Masterson, Brandi W. Hilder, Jaszianne Tolbert, Thomas Renaud, Christoph Heuck, and Chalmer Tomlinson are employees of Johnson & Johnson and may hold stock, stock options, or equity in Johnson & Johnson. Philippe Moreau has received honoraria from and is a member of an advisory board for AbbVie, Amgen, BMS/Celgene, GSK, Janssen Biotech, Johnson & Johnson, Pfizer, Sanofi, and Takeda. Jesús San‐Miguel is a consultant and advisory board member for AbbVie, Amgen, BMS/Celgene, GSK, HaemaLogiX, Johnson & Johnson, Karyopharm, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Roche, Sanofi, Secura Bio, and Takeda. Paula Rodríguez‐Otero is a consultant for AbbVie, BMS, H3 Biomedicine, Janssen Global Services, Pfizer, Roche, and Stemline; is a member of a board of directors or advisory committee for AbbVie, BMS, GSK, Johnson & Johnson, Kite Pharma, Oncopeptides, Pfizer, Sanofi, and Takeda; and has received honoraria from Amgen, BMS, GSK, Janssen Pharmaceuticals, Johnson & Johnson, Regeneron, Sanofi, and Takeda. Ajai Chari is a consultant for Adaptive Biotechnologies, Amgen, Antengene, BMS, Janssen Pharmaceuticals, Johnson & Johnson, Millenium/Takeda, and Secura Bio; is a member of an advisory board for AbbVie, Amgen, BMS, Genentech, GSK, Johnson & Johnson, Karyopharm, Sanofi, Seattle Genetics, Secura Bio, and Shattuck Labs; and has received research funding from Amgen, BMS, Johnson & Johnson, Millenium/Takeda, and Seattle Genetics. The other author declares no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

