An observational study of the effectiveness and safety of nivolumab plus chemotherapy for untreated advanced or recurrent gastric cancer in Japanese real-world settings: the G-KNIGHT study
- PMID: 40632416
- PMCID: PMC12378153
- DOI: 10.1007/s10120-025-01641-7
An observational study of the effectiveness and safety of nivolumab plus chemotherapy for untreated advanced or recurrent gastric cancer in Japanese real-world settings: the G-KNIGHT study
Abstract
Background: Nivolumab plus chemotherapy has shown efficacy in clinical trials for advanced or recurrent gastric cancer (GC). However, real-world utilization data are limited. In this study, we aimed to assess the effectiveness, safety, and treatment status of first line nivolumab plus chemotherapy in Japanese patients with treatment-naïve advanced or recurrent GC.
Methods: Untreated patients with advanced or recurrent GC who initiated nivolumab plus chemotherapy as first line treatment from November 2021 to June 2023 across 23 Japanese sites were enrolled in this observational study (G-KNIGHT). This report focused on the objective response rate (ORR), real-world progression-free survival (rwPFS), and the treatment-related adverse event (TRAE) incidence. Furthermore, subgroup analyses for ORR and rwPFS were conducted for patients stratified by various factors including age and the programmed cell death ligand 1 (PD-L1) combined positive score (CPS).
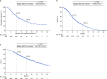
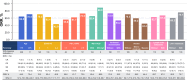
Results: Among 527 patients (median age, 70.3 years; 25.2% aged ≥ 75 years; 65.5% male; 84.3% with advanced GC), the median follow-up period was 10.4 (interquartile range, 6.7-15.2) months. The ORR was 65.6% (95% confidence interval [CI], 59.9-70.9%). The median rwPFS (months, 95% CI) was 6.9 (6.2-7.6); by subgroups: age < 75 years, 6.7 (6.0-7.5); age ≥ 75 years, 7.4 (6.2-8.6); PD-L1 CPS < 1, 7.5 (6.5-9.0); CPS 1-5, 6.2 (5.5-8.0); and CPS ≥ 5, 7.0 (6.2-8.2). TRAEs occurred in 91.3% of patients, with 40.4% experiencing grade ≥ 3 events.
Conclusions: This large-scale real-world study supports the effectiveness and safety of nivolumab plus chemotherapy in untreated Japanese patients with advanced or recurrent GC.
Keywords: Japan; Nivolumab; Observational study; Stomach neoplasms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Shigenori Kadowaki has received research funding from Ono Pharmaceutical Co. Ltd., MSD K.K., Janssen Pharmaceutical K.K., Eli Lilly Japan K.K., DAIICHI SANKYO COMPANY, LIMITED, AstraZeneca K.K., TAIHO PHARMACEUTICAL, CO., LTD., Nobelpharma Co., Ltd., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., and AbbVie GK; and honoraria from Ono Pharmaceutical Co. Ltd., MSD K.K., Merck KgaA, Eli Lilly Japan K.K., Bayer Yakuhin, Ltd., Novartis Pharma K.K., TAIHO PHARMACEUTICAL, CO., LTD., DAIICHI SANKYO COMPANY, LIMITED, Bristol Myers Squibb, Chugai Pharmaceutical Co., Ltd., and Eisai Co., Ltd. Tomoyuki Otsuka has received honoraria from Bristol Myers Squibb and Ono Pharmaceutical Co. Ltd. Keiko Minashi has received research funding from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., TAIHO PHARMACEUTICAL, CO., LTD., DAIICHI SANKYO COMPANY, LIMITED, and MSD K.K.; and honoraria from Bristol Myers Squibb and Ono Pharmaceutical Co. Ltd. Shinichi Nishina has received from Bristol Myers Squibb and Ono Pharmaceutical Co. Ltd. Hiroshi Yabusaki has no conflict of interest. Chiaki Inagaki has received honoraria from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., DAIICHI SANKYO COMPANY, LIMITED, Takeda Pharmaceutical Co., Ltd., and Astellas Pharma Inc. Tomohiro Nishina has received honoraria from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., TAIHO PHARMACEUTICAL, CO., LTD., Eli Lilly Japan K.K., DAIICHI SANKYO COMPANY, LIMITED, Astellas Pharma Inc., and MSD K.K. Hisateru Yasui has received research funding from Ono Pharmaceutical Co. Ltd.; and honoraria from Bristol Myers Squibb, TAIHO PHARMACEUTICAL, CO., LTD., Chugai Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. Hiroshi Matsuoka has received honoraria from Bristol Myers Squibb and DAIICHI SANKYO COMPANY, LIMITED. Nozomu Machida has received honoraria from Bristol Myers Squibb and Ono Pharmaceutical Co. Ltd. Masahiro Tsuda has received honoraria from Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd., Bristol Myers Squibb, and MSD K.K. Fumio Nagashima has received honoraria from TAIHO PHARMACEUTICAL, CO., LTD., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., DAIICHI SANKYO COMPANY, LIMITED, Merck Biopharma Co., Ltd., Janssen Pharmaceutical K.K., and Takeda Pharmaceutical Co., Ltd. Hisashi Hosaka has no conflict of interest. Junichi Matsubara has no conflict of interest. Hiroyuki Arai has received research funding from Novartis Pharma K.K. and honoraria from Bristol Myers Squibb. Satoshi Ida has no conflict of interest. Yuya Kimijima is an employee of Bristol Myers Squibb. Yuko Matsuda is an employee of Ono Pharmaceutical Co. Ltd. Manabu Muto has received research funding from Ono Pharmaceutical Co. Ltd. and Meiji Seika Pharma Co., Ltd.; and honoraria from Chugai Pharmaceutical Co., Ltd., MSD K.K., AstraZeneca K.K., Ono Pharmaceutical Co. Ltd., and Meiji Seika Pharma Co., Ltd. Kei Muro has received research funding from Amgen Inc., Ono Pharmaceutical Co. Ltd., Astellas Pharma Inc., Sanofi K.K., TAIHO PHARMACEUTICAL, CO., LTD., PRA Health Sciences, Inc., Parexel International Inc., Novartis Pharma K.K., Chugai Pharmaceutical Co., Ltd., and MSD K.K.; consulting fees from Amgen Inc., AstraZeneca K.K., Ono Pharmaceutical Co. Ltd., Astellas Pharma Inc., and Chugai Pharmaceutical Co., Ltd.; honoraria from Ono Pharmaceutical Co. Ltd., TAIHO PHARMACEUTICAL, CO., LTD., Bristol Myers Squibb, Eli Lilly Japan K.K., MSD K.K., Takeda Pharmaceutical Co., Ltd., and DAIICHI SANKYO COMPANY, LIMITED; and served as a member of the Data Safety Monitoring Board or Advisory Board for Astellas Pharma Inc., Amgen Inc., AstraZeneca K.K., and Takeda Pharmaceutical Co., Ltd. Ethics approval: This study was approved by Non-Profit Organization MINS Research Ethics Committee (MINS-REC-220205) and was conducted in compliance with the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects and the Act on the Protection of Personal Information. All study procedures were conducted in accordance with the principles of the World Medical Association Declaration of Helsinki. Consent to participate: The authors thank the patients and families who are making the study possible and the investigators at the centers participating in the study. The authors also thank the clinical study teams. The data system was provided by Prime Research Institute for Medical RWD, Inc. Writing and editorial assistance was provided by Mebix, Inc., funded by Bristol Myers Squibb and Ono Pharmaceutical Co., Ltd.
Figures

References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- Carioli G, Malvezzi M, Bertuccio P, Hashim D, Waxman S, Negri E, et al. Cancer mortality in the elderly in 11 countries worldwide, 1970–2015. Ann Oncol. 2019;30:1344–55. - PubMed
-
- Cancer Statistics. Cancer information service, national cancer center, Japan. 2024. https://ganjoho.jp/reg_stat/statistics/data/dl/excel/cancer_incidenceNCR...; https://ganjoho.jp/reg_stat/statistics/data/dl/excel/cancer_mortality(19... (accessed May 26, 2025).
-
- Sekiguchi M, Oda I, Matsuda T, Saito Y. Epidemiological trends and future perspectives of gastric cancer in Eastern Asia. Digestion. 2022;103:22–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

