Sustained Accumulation of Molecular Clock Suppressors Period 1 and Period 2 Promotes C2C12 Myotube Atrophy Through an Autocrine-Mediated Mechanism With Relevance to Androgen Deprivation-Induced Limb Muscle Mass Loss
- PMID: 40632504
- PMCID: PMC12316099
- DOI: 10.1093/function/zqaf030
Sustained Accumulation of Molecular Clock Suppressors Period 1 and Period 2 Promotes C2C12 Myotube Atrophy Through an Autocrine-Mediated Mechanism With Relevance to Androgen Deprivation-Induced Limb Muscle Mass Loss
Abstract
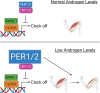
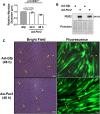
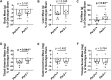
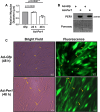
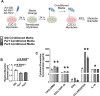
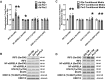
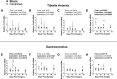
Low testosterone in males (hypogonadism) is associated with limb muscle mass loss, yet the underlying mechanisms of muscle mass loss remain largely unknown. We previously showed androgen deprivation disrupted limb muscle molecular clock function, and the disruption coincided with elevated levels of the primary molecular clock suppressor, Period 2 (Per2). The purposes herein were to determine if PER2 overexpression leads to muscle atrophy and if preventing PER2 accumulation blunts limb muscle mass loss in response to androgen deprivation. Here, we identify Per2 as a negative regulator of muscle size. Overexpression of Per2 in differentiated C2C12 myotubes reduced myotube diameter, while deletion of Per2 in male mice partially preserved tibialis anterior (TA) mass following castration. The muscle-sparing effect of Per2 deletion in vivo was specific to the TA despite evidence of molecular clock disruption and mass loss in other muscles. Subsequently, we show overexpression of the other primary clock suppressor, Period 1 (Per1) also reduced myotube diameter in differentiated C2C12 myotubes. Mechanistically, both Per1 and Per2 overexpression in vitro induced muscle atrophy in part by an autocrine-mediated mechanism likely involving inflammation as their overexpression induced an inflammatory gene expression signature and increased cytokine/chemokine secretion. Moreover, incubation of C2C12 myotubes in the media conditioned from Per1 or Per2 overexpressing myotubes reduced myotube diameter. Several inflammatory genes identified in vitro were also altered in the limb muscles in response to androgen deprivation. These findings identify a previously unrecognized role for Per1/2 in regulating skeletal muscle mass with implications for muscle loss during hypogonadism.
Keywords: RNA sequencing; circadian rhythm; hypogonadism; inflammation.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–195. - PubMed
-
- Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76(2):378–383. - PubMed
-
- Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metabol. 2003;88(1):358–362. - PubMed
-
- Ferrando AA, Sheffield-Moore M, Yeckel CW et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282(3):E601–E607. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
