Ixekizumab and Malignant Neoplasms: A Pooled Analysis of Data From 25 Randomized Clinical Trials
- PMID: 40632525
- PMCID: PMC12242818
- DOI: 10.1001/jamadermatol.2025.2056
Ixekizumab and Malignant Neoplasms: A Pooled Analysis of Data From 25 Randomized Clinical Trials
Abstract
Importance: Assessing malignant neoplasm risk among patients with long-term biologic exposure is of interest. This study provides insight into the risk of malignant neoplasm among patients with psoriasis (PsO), psoriatic arthritis (PsA), or axial spondyloarthritis (axSpA) who received ixekizumab (IXE) over time.
Objective: To determine the incidence of malignant neoplasms among patients with PsO, PsA, or axSpA who received long-term (up to 6 years) IXE treatment, and to compare the recorded incidences (excluding nonmelanoma skin cancer) with those observed in the US general population.
Design, setting, and participants: This multicenter, global pooled analysis examined patient data from 25 randomized clinical trials (RCTs) in patients with PsO, PsA, or axSpA receiving at least 1 dose of IXE over 5 years (PsO) or 3 years (PsA and axSpA). Eligibility criteria varied across the 25 RCTs, but most of the patients were naive to biologic treatments. The primary analysis was performed in March 2021 (PsA) and March 2022 (PsO and axSpA).
Intervention: Long-term treatment with IXE.
Main outcomes and measures: Incidence rates of malignant neoplasms and standardized incidence ratios (SIRs).
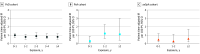
Results: The mean age across the 3 indications was 45.9 years; most patients in the PsO and axSpA cohorts were male (4696/6892 [68.1%] and 650/932 [69.7%], respectively), whereas the proportion of male to female patients in the PsA cohort was largely balanced (679/1401 [48.5%] vs 722/1401 [51.5%], respectively). The study included 6892 patients with PsO, 1401 patients with PsA, and 932 patients with axSpA, representing a cumulative exposure to IXE of 22 371.1 patient-years (PY) (18 025.7 PY for PsO, 2247.7 PY for PsA, and 2097.7 PY for axSpA). Malignant neoplasms were reported among 141 patients with PsO (2.0%; incidence rate [IR], 0.8 per 100 PY [95% CI, 0.7-0.9]), 15 patients with PsA (1.1%; IR, 0.7 per 100 PY [95% CI, 0.4-1.1]), and 9 patients with axSpA (1.0%; IR, 0.4 per 100 PY [95% CI, 0.2-0.8]). IRs of malignant neoplasms at 1-year intervals remained low (≤1.2 per 100 PY) and constant over time. SIRs with 95% CIs were below or near 1 (PsO, 0.89 [95% CI, 0.71-1.08]; PsA, 0.49 [95% CI, 0.13-0.85]; axSpA, 1.07 [95% CI, 0.37-1.77]).
Conclusions and relevance: This pooled analysis of 25 RCTs demonstrated that the safety profile of IXE supports long-term use in patients with PsO, PsA, or axSpA. This is evidenced by incidences of malignant neoplasms consistent with previous reports, and with SIRs of malignant neoplasms across indications similar to the US general population.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

