Mycophenolic acid treatment drives the emergence of novel SARS-CoV-2 variants
- PMID: 40632557
- PMCID: PMC12280948
- DOI: 10.1073/pnas.2500276122
Mycophenolic acid treatment drives the emergence of novel SARS-CoV-2 variants
Abstract
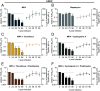
Mycophenolic acid (MPA) is commonly used in immunosuppressive regimens following solid organ transplantation. We demonstrate that MPA treatment reproducibly inhibits the replication of a range of viruses, including severe respiratory syndrome coronavirus 2 (SARS-CoV-2). Mechanistically, we identified cellular guanosine triphosphate pool depletion as a key mediator of this antiviral effect. Strikingly, this inhibition can be overcome which was correlated with the emergence of three breakthrough mutations in the SARS-CoV-2 genome (S P812R, ORF3 Q185H, and E S6L). Subsequent analyses confirmed that the combination of these mutations conferred accelerated replication kinetics, higher viral titers, and more rapid onset of cytopathic effects, but not MPA resistance. Comparison of global transcriptional responses to infection highlighted dysregulation of specific cellular gene programs under MPA treatment prior to breakthrough mutation emergence. Together, these findings identify viral and host drivers of variant emergence under immunosuppression. They also advocate for close monitoring of immunosuppressed patients, where emergence of novel viral variants with a fitness advantage may arise.
Keywords: SARS-CoV-2; guanosine triphosphate (GTP) depletion; immunosuppression; mycophenolic acid; novel variants.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Burrell C. J., Howard C. R., Murphy F. A., “Epidemiology of viral infections” in Fenner and White’s Medical Virology, Burrell C. J., Howard C. R., Murphy F. A., Eds. (Elsevier/AP Academic Press is an imprint of Elsevier, Amsterdam, Boston, ed. 5, 2017), pp. 185–203.
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

