Effect of CYP3A4 Methylation on Tacrolimus Pharmacokinetics
- PMID: 40632895
- PMCID: PMC12588635
- DOI: 10.1097/FTD.0000000000001351
Effect of CYP3A4 Methylation on Tacrolimus Pharmacokinetics
Abstract
Background: Common genetic variants in CYP3A4 together only explain a limited amount of the variability in tacrolimus clearance. This cross-sectional study aimed to explore the extent to which pharmacokinetic variability can be explained by methylation of the CYP3A4 gene.
Methods: Residual tissue material from liver biopsies routinely collected 6 months post-transplantation was used. Inclusion criteria were tacrolimus once daily (Advagraf) in a steady state (ie, no dose change in the previous 3 days); assessment of tacrolimus pharmacokinetics within 3 weeks of the biopsy; and no documented episode of rejection for at least 3 months prior. Patients and liver donor tissue were genotyped. Only patients in which the patient and the donor had a genotype that did not express the CYP3A5 protein were included. The liver biopsy tissue material was then analyzed using an Illumina Infinium MethylationEPIC array.
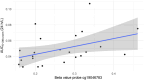
Results: Of the 28 patients who met the inclusion criteria, 23 passed the quality control assessment required for the methylation analysis. Increased methylation of 1 of the 10 methylation probes within the CYP3A4 gene region (cg19046783) was positively correlated (Spearman correlation coefficient, 0.52) with the dose-normalized area under the concentration versus time curve (AUC) 0-24h ( P = 0.01). When quantified using univariate linear regression, this probe explained 18% of the variation in the dose-normalized AUC 0-24h . Interestingly, cg19046783 had the lowest mean methylation and highest biological variation.
Conclusions: Increased methylation of 1 of the 10 methylation probes within the CYP3A4 gene region (cg19046783) was positively correlated with increased dose-normalized AUC 0-24h , which explained 18% of the variation in the dose-normalized AUC 0-24h using univariate linear regression.
Keywords: liver; pharmacokinetics; tacrolimus; therapeutic drug monitoring; transplantation.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures
References
-
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623–653. - PubMed
-
- Brunet M, van Gelder T, Åsberg A, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

