Risk Assessment With Ultra-Low-Pass Whole-Genome Sequencing of Cell-Free DNA for Large B-Cell Lymphoma
- PMID: 40632977
- PMCID: PMC12258806
- DOI: 10.1200/PO-25-00200
Risk Assessment With Ultra-Low-Pass Whole-Genome Sequencing of Cell-Free DNA for Large B-Cell Lymphoma
Abstract
Purpose: Although deep targeted DNA sequencing of liquid biopsies has shown prognostic utility in large B-cell lymphoma (LBCL), the routine clinical adoption of these assays remains limited because of their high costs.
Materials and methods: Here, leveraging a well-annotated cohort encompassing both frontline and relapsed/refractory (R/R) LBCL, we profiled patient plasma samples with two complementary modalities-ultra-low-pass whole-genome sequencing (ULP-WGS) and deep targeted DNA sequencing, the former being a cost-effective method to profile large scale chromosomal abnormalities and estimate tumor burden.
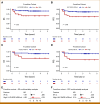
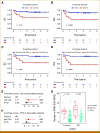
Results: Our findings revealed a strong association of high cell-free tumor burden by both genomic profiling modalities with established measures of tumor burden and patient survival. Notably, the associations with survival remained statistically significant after accounting for international prognostic index scoring. Furthermore, we showed that del(17p) in circulating tumor DNA as detected by ULP-WGS was strongly associated with TP53 mutation status and predicted for significantly inferior outcome in frontline LBCL patients but not in patients with R/R LBCL.
Conclusion: Our study demonstrates that ULP-WGS can provide robust prognostic biomarkers for both frontline and R/R LBCL, highlighting its broad applicability for risk stratification.
Trial registration: ClinicalTrials.gov NCT03702309.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Tavakkoli M, Barta SK: 2024 update: Advances in the risk stratification and management of large B-cell lymphoma. Am J Hematol 98:1791-1805, 2023 - PubMed
-
- Meriranta L, Alkodsi A, Pasanen A, et al. : Molecular features encoded in the ctDNA reveal heterogeneity and predict outcome in high-risk aggressive B-cell lymphoma. Blood 139:1863-1877, 2022 - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

