Single-Agent Divarasib in Patients With KRAS G12C-Positive Non-Small Cell Lung Cancer: Long-Term Follow-Up of a Phase I Study
- PMID: 40632992
- PMCID: PMC12527784
- DOI: 10.1200/JCO-25-00040
Single-Agent Divarasib in Patients With KRAS G12C-Positive Non-Small Cell Lung Cancer: Long-Term Follow-Up of a Phase I Study
Abstract
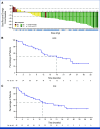
Divarasib (GDC-6036), an oral, highly potent and selective next-generation KRAS G12C inhibitor, has demonstrated a manageable safety profile and promising antitumor activity in patients with advanced KRAS G12C-positive non-small cell lung cancer (NSCLC). Here, we report long-term (≥1 year) follow-up of single-agent divarasib from the ongoing, open-label, and multicenter phase I study (ClinicalTrials.gov identifier: NCT04449874). The primary objective was safety, and the other objectives included preliminary antitumor activity. Overall, 65 patients with advanced KRAS G12C-positive NSCLC received single-agent oral divarasib 50-400 mg once daily and 31 patients (48%) were treated beyond 1 year. Divarasib continued to be well tolerated, and the safety profile beyond 1 year was consistent with the overall safety profile. In patients with measurable disease at baseline across all dose levels (n = 63), the confirmed objective response rate was 55.6% (95% CI, 42.5 to 68.1), and the median duration of response was 18.0 months (95% CI, 11.1 to 24.9). The median progression-free survival was 13.8 months (95% CI, 9.8 to 25.4) in the overall population (N = 65) and 15.3 months (95% CI, 12.3 to 26.1) among patients assigned to the 400-mg dose level (n = 44). With extended follow-up, divarasib demonstrated long-term safety and antitumor activity in patients with advanced KRAS G12C-positive NSCLC.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384:185–187. - PubMed
-
- de Langen AJ, Johnson ML, Mazieres J, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: A randomised, open-label, phase 3 trial. Lancet. 2023;401:733–746. - PubMed
-
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a G12C mutation. N Engl J Med. 2022;387:120–131. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

