SENP1-Sirt3 axis regulates type II alveolar epithelial cell activity to confer resistance against oxidative damage in lung tissue
- PMID: 40633428
- PMCID: PMC12274925
- DOI: 10.1016/j.redox.2025.103752
SENP1-Sirt3 axis regulates type II alveolar epithelial cell activity to confer resistance against oxidative damage in lung tissue
Abstract
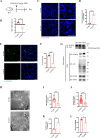
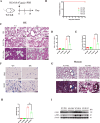
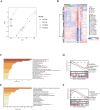
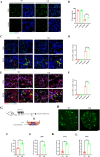
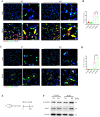
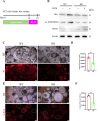
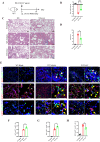
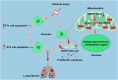
Oxidative damage exacerbates pulmonary fibrosis by impairing alveolar type II epithelial (AT2) cell function. This study demonstrates that the SUMO-specific protease 1 (SENP1)-Sirtuin 3 (Sirt3) axis, critical for mitochondrial redox regulation, is suppressed in AT2 cells during lung injury. In bleomycin-induced pulmonary fibrosis models, activating the SENP1-Sirt3 axis via Sirt3 SUMOylation site mutation (Sirt3 K223R) reduced Superoxide Dismutase 2 (SOD2) acetylation, thereby lowering mitochondrial reactive oxygen species (mtROS) accumulation and apoptosis. This intervention increased AT2 cell proliferation and differentiation into alveolar type I cells while reducing Keratin 8 (KRT8)+ transitional cell number, a profibrotic population. Additionally, SENP1-Sirt3 activation attenuated inflammation and fibrosis in lung tissue. Transcriptomic analysis linked the axis to enhanced Wnt signaling and lipid metabolism pathways, promoting AT2 stemness. Antioxidant N-acetylcysteine (NAC) supplementation mirrored these benefits, reinforcing ROS clearance as a therapeutic mechanism. These findings highlight SENP1-Sirt3 as a pivotal regulator of AT2 resilience, offering a potential strategy to mitigate fibrosis by targeting mitochondrial oxidative stress and cellular plasticity.
Keywords: AT2 cell; Lung fibrosis; Mitochondria; Oxidative damage; SENP1-Sirt3 axis.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jinke Cheng reports financial support was provided by the National Key Research and Development Program of China. Jinke Cheng reports financial support was provided by National Natural Science Foundation of China. Tianshi Wang reports financial support was provided by National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Deficient AMPK-SENP1-Sirt3 signaling impairs mitochondrial complex I function in Parkinson's disease model.Transl Neurodegener. 2025 Jul 1;14(1):34. doi: 10.1186/s40035-025-00489-2. Transl Neurodegener. 2025. PMID: 40597361 Free PMC article.
-
Enteral nutrition intervention improves intestinal ischemia-reperfusion injury by modulating the SENP1/SIRT3 axis.J Clin Biochem Nutr. 2025 Jul;77(1):55-63. doi: 10.3164/jcbn.25-31. Epub 2025 Apr 9. J Clin Biochem Nutr. 2025. PMID: 40777823 Free PMC article.
-
Mitochondria-Targeting SIRT3 Activator Effectively Controls Bleomycin-Induced Pulmonary Fibrosis.Biofactors. 2025 Jul-Aug;51(4):e70032. doi: 10.1002/biof.70032. Biofactors. 2025. PMID: 40579906
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
-
Function of intramitochondrial melatonin and its association with Warburg metabolism.Cell Signal. 2025 Jul;131:111754. doi: 10.1016/j.cellsig.2025.111754. Epub 2025 Mar 21. Cell Signal. 2025. PMID: 40122433 Review.
References
-
- Wu H., Yu Y., Huang H., Hu Y., Fu S., Wang Z., Shi M., Zhao X., Yuan J., Li J., Yang X., Bin E., Wei D., Zhang H., Zhang J., Yang C., Cai T., Dai H., Chen J., Tang N. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. 2020;180:107–121 e117. - PubMed
-
- Strunz M., Simon L.M., Ansari M., Kathiriya J.J., Angelidis I., Mayr C.H., Tsidiridis G., Lange M., Mattner L.F., Yee M., Ogar P., Sengupta A., Kukhtevich I., Schneider R., Zhao Z., Voss C., Stoeger T., Neumann J.H.L., Hilgendorff A., Behr J., O'Reilly M., Lehmann M., Burgstaller G., Konigshoff M., Chapman H.A., Theis F.J., Schiller H.B. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020;11:3559. - PMC - PubMed
-
- Enomoto Y., Katsura H., Fujimura T., Ogata A., Baba S., Yamaoka A., Kihara M., Abe T., Nishimura O., Kadota M., Hazama D., Tanaka Y., Maniwa Y., Nagano T., Morimoto M. Autocrine TGF-beta-positive feedback in profibrotic AT2-lineage cells plays a crucial role in non-inflammatory lung fibrogenesis. Nat. Commun. 2023;14:4956. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

