Therapeutic targeting of FOSL1 and RELA-dependent transcriptional mechanisms to suppress pancreatic cancer metastasis
- PMID: 40634284
- PMCID: PMC12241458
- DOI: 10.1038/s41419-025-07810-x
Therapeutic targeting of FOSL1 and RELA-dependent transcriptional mechanisms to suppress pancreatic cancer metastasis
Abstract
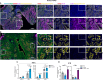
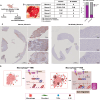
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer often diagnosed at an advanced stage, leading to a poor prognosis. The tumor microenvironment (TME) plays a crucial role in driving metastasis, with inflammatory signaling pathways contributing to tumor progression and therapy resistance. However, the combined effects of inflammatory and oncogenic signaling on the epigenetic regulation of PDAC metastasis are poorly understood. Here, we demonstrate that tumor necrosis factor-alpha (TNFα) and epidermal growth factor (EGF) signaling converge to regulate PDAC cell migration through the activation of NF-κB and AP-1 transcription factors. Using single-cell RNA sequencing, in vitro and in vivo models, we show that the simultaneous activation of these pathways with TNFα and EGF cooperatively induces the expression of genes associated with cell motility and migration. Consistently, combinatorial induced genes are co-regulated by the transcription factors FOSL1 and RELA. Remarkably, inhibition of NF-κB transcriptional activity with a glucocorticoid receptor (GR) mixed agonist significantly reduced PDAC cell migration by decreasing RNA polymerase II recruitment to target genes. These findings reveal a novel mechanism by which inflammatory and oncogenic pathways cooperate to drive PDAC metastasis and highlight the therapeutic potential of GR agonists in mitigating tumor cell migration. Our study offers promising avenues for developing mechanism-based therapeutic strategies in PDAC management.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The author declare no competing interests. Compliance with ethics statement: This study received all necessary ethical approvals from the Institutional Review Board (IRB) under protocols [354-06, 66-06, and 19-012104], and from the Institutional Animal Care and Use Committee (IACUC) under protocol [A00003954-18-R24]. All experimental procedures and methods were conducted in accordance with relevant institutional guidelines and regulations. Informed consent was obtained from all human participants or their legal guardians prior to inclusion in the study, in accordance with IRB-approved protocols and institutional standards.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

