Spatial variation of skin-associated microbiota in a green salamander metapopulation
- PMID: 40634333
- PMCID: PMC12241389
- DOI: 10.1038/s41598-025-05305-5
Spatial variation of skin-associated microbiota in a green salamander metapopulation
Abstract
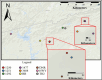
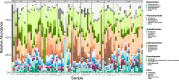
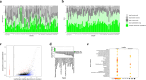
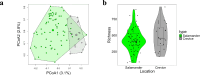
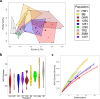
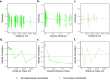
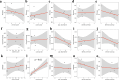
As compared to free-living microbes, host-associated (HA) microbes are unique in that they experience dispersal at both the microbial and host scales. This is particularly clear in systems where hosts experience strong barriers to dispersal, for example hosts that live in patchy habitats or metapopulations. In these systems, there are both limits to dispersal of HA microbes from host to host (microbial scale dispersal) and limits to dispersal of hosts from habitat patch to habitat patch (host scale dispersal). Few studies have considered how host and microbial scale dispersal limitation impacts spatial patterns in HA microbiota. We address this question using green salamander skin microbiota. This species exhibits population structure wherein animals primarily inhabit disjunct rock outcrops with occasional dispersal between outcrop populations. We find strong evidence for the importance of host scale dispersal based on differences in distance-decay of similarity between salamander and environmental microbiota. We find weaker evidence/mixed support for the importance of microbial scale dispersal based on low similarity of the host exclusive skin microbiota but a lack of dependence of skin microbiota similarity and diversity on host density. We discuss implications of our findings, both with reference to other processes governing HA microbiota assembly and with reference to amphibian conservation. For the latter, we consider variation in chytrid-inhibitory community profiles across populations and potential ramifications in terms of variation in susceptibility to chytridiomycosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- McCoy, K. D., Ronchi, F. & Geuking, M. B. Host-microbiota interactions and adaptive immunity. Immunol. Rev.279, 63–69 (2017). - PubMed
-
- Ezenwa, V. O., Gerardo, N. M., Inouye, D. W., Medina, M. & Xavier, J. B. Animal behavior and the microbiome. Science338, 198–199. 10.1126/science.1227412 (2012). - PubMed
-
- Lewis, Z. & Lizé, A. Insect behaviour and the microbiome. Curr. Opin. Insect Sci.9, 86–90. 10.1016/j.cois.2015.03.003 (2015). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

