The role of intestinal microbiota in the humoral response to SARS-CoV-2 after mRNA-1273 vaccination
- PMID: 40634526
- PMCID: PMC12241598
- DOI: 10.1038/s41598-025-11103-w
The role of intestinal microbiota in the humoral response to SARS-CoV-2 after mRNA-1273 vaccination
Abstract
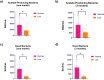
The gut microbiota plays a key role in shaping immune responses, including those induced by vaccination. Its impact on the humoral response to mRNA-based SARS-CoV-2 vaccines, however, remains underexplored. We analyzed gut microbiota composition and anti-Spike (S) IgG levels in 50 healthcare workers vaccinated with the mRNA-1273 SARS-CoV-2 vaccine. Participants were stratified into low, medium, and high responders based on IgG titers 30 days post-vaccination. Stool samples were collected at baseline, and 16 S rRNA sequencing was used to assess microbiota diversity and taxonomic profiles. Alpha diversity indices showed no significant differences across response groups. However, specific microbial signatures were associated with vaccine response. Higher relative abundance of Clostridia, Clostridiales, Ruminococcaceae, and Odoribacter splanchnicus correlated with stronger IgG responses. Functional microbiome analysis revealed enrichment of acetate-producing pathways in high responders (p = 0.012), suggesting a role for short-chain fatty acids in enhancing vaccine-induced immunity. Logistic regression and Random Forest models identified these taxa as predictors of strong antibody responses. The area under the ROC curve (AUC) for individual taxa ranged from 0.70 to 0.76, indicating moderate predictive performance. Conversely, taxa such as Hallella and Sutterella wadsworthensis were linked to lower responses. These findings support a modulatory role of the gut microbiota in mRNA vaccine immunogenicity and highlight microbial metabolic functions as potential targets to boost vaccine efficacy in personalized immunization strategies.
Keywords: 16S rRNA sequencing; Bacterial taxa and immunogenicity; Gut microbiota; MRNA-1273; Microbiome and immunity; SARS-CoV-2 vaccination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Haas, E. J. et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in israel: an observational study using National surveillance data. Lancet397, 1819–1829 (2021). - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

