Coenzyme Q headgroup intermediates can ameliorate a mitochondrial encephalopathy
- PMID: 40634618
- PMCID: PMC12422950
- DOI: 10.1038/s41586-025-09246-x
Coenzyme Q headgroup intermediates can ameliorate a mitochondrial encephalopathy
Abstract
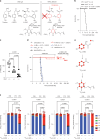
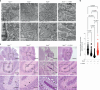
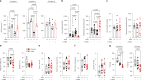
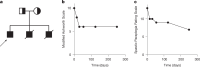
Decreased brain levels of coenzyme Q10 (CoQ10), an endogenously synthesized lipophilic antioxidant1,2, underpin encephalopathy in primary CoQ10 deficiencies3,4 and are associated with common neurodegenerative diseases and the ageing process5,6. CoQ10 supplementation does not increase CoQ10 pools in the brain or in other tissues. The recent discovery of the mammalian CoQ10 headgroup synthesis pathway, in which 4-hydroxyphenylpyruvate dioxygenase-like protein (HPDL) makes 4-hydroxymandelate (4-HMA) to synthesize the CoQ10 headgroup precursor 4-hydroxybenzoate (4-HB)7, offers an opportunity to pharmacologically restore CoQ10 synthesis and mechanistically treat CoQ10 deficiencies. To test whether 4-HMA or 4-HB supplementation promotes CoQ10 headgroup synthesis in vivo, here we administered 4-HMA and 4-HB to Hpdl-/- mice, which model an ultra-rare, lethal mitochondrial encephalopathy in humans. Both 4-HMA and 4-HB were incorporated into CoQ9 and CoQ10 in the brains of Hpdl-/- mice. Oral treatment of Hpdl-/- pups with 4-HMA or 4-HB enabled 90-100% of Hpdl-/- mice to live to adulthood. Furthermore, 4-HB treatment stabilized and improved the neurological symptoms of a patient with progressive spasticity due to biallelic HPDL variants. Our work shows that 4-HMA and 4-HB can modify the course of mitochondrial encephalopathy driven by HPDL variants and demonstrates that CoQ10 headgroup intermediates can restore CoQ10 synthesis in vivo.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.S., Q.S., R.S.B. and M.E.P. are co-inventors on patents related to the use of 4-HMA, 4-HB and analogues in the diagnosis and treatment of neurodevelopmental and other diseases assigned to New York University. M.E.P. directly supervised the preclinical research. The treatment was conducted and supervised by C.M. and G.M.R. under an institutional conflict-of-interest management plan designed and implemented by NYU Langone Health in accordance with its policies. M.E.P. consulted on the clinical protocol and C.M. and G.M.R. directed the course of treatment in accordance with the institutional plan. Financial support for the clinical treatment was provided by the Pershing Square Foundation. The other authors declare no competing interests.
Figures









References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

