Real-world deployment of a fine-tuned pathology foundation model for lung cancer biomarker detection
- PMID: 40634781
- PMCID: PMC12443599
- DOI: 10.1038/s41591-025-03780-x
Real-world deployment of a fine-tuned pathology foundation model for lung cancer biomarker detection
Abstract
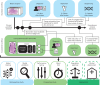
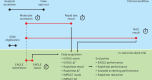
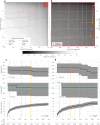
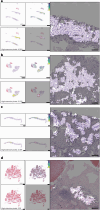
Artificial intelligence models using digital histopathology slides stained with hematoxylin and eosin offer promising, tissue-preserving diagnostic tools for patients with cancer. Despite their advantages, their clinical utility in real-world settings remains unproven. Assessing EGFR mutations in lung adenocarcinoma demands rapid, accurate and cost-effective tests that preserve tissue for genomic sequencing. PCR-based assays provide rapid results but with reduced accuracy compared with next-generation sequencing and require additional tissue. Computational biomarkers leveraging modern foundation models can address these limitations. Here we assembled a large international clinical dataset of digital lung adenocarcinoma slides (N = 8,461) to develop a computational EGFR biomarker. Our model fine-tunes an open-source foundation model, improving task-specific performance with out-of-center generalization and clinical-grade accuracy on primary and metastatic specimens (mean area under the curve: internal 0.847, external 0.870). To evaluate real-world clinical translation, we conducted a prospective silent trial of the biomarker on primary samples, achieving an area under the curve of 0.890. The artificial-intelligence-assisted workflow reduced the number of rapid molecular tests needed by up to 43% while maintaining the current clinical standard performance. Our retrospective and prospective analyses demonstrate the real-world clinical utility of a computational pathology biomarker.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.V. and T.J.F. report intellectual property rights and equity interest in Paige.AI, Inc. T.J.F. is employed by Eli Lilly. F.R.H. has acted as an adviser of Amgen, AstraZeneca, Bicara Therapeutics, BMS, Daiichi, G1 Therapeutics, Genentech/Roche, Genzyme/Sanofi, GSK, Merck, Merus Therapeutics, Nectin Therapeutics, NextCure, Novartis, OncoCyte, Oncohost and Regeneron. H.Y. has received consulting fees from AstraZeneca, Blueprint Medicines, Daiichi Sankyo, Genentech/Roche, Janssen, Merus, Pfizer, Regeneron, Ribon Therapeutics and Turning Point Therapeutics. M.H. holds fiduciary roles with the International Society of Bone and Soft Tissue Pathology and the United States & Canadian Academy of Pathology (USCAP). M.A. holds a fiduciary role with the Association for Molecular Pathology, has provided professional services for Biocartis US, Inc. and PER Events, LLC, and has intellectual property rights associated with SOPHiA. A.E. declares research grant support from Kanvas Bioscience, GMT Bioscience, BMS, Merck and Astrazeneca, and consulting and honoraria from BMS, Merck, Astrazeneca and EMD SorenoGENETICS. All disclosed competing interests are outside of the submitted work. G.C., N.K., S.N., S.S., E.F., R.K., S.M., N.P., P.J.S., I.H., N.N., L.M.A., A.B., T.J., M.R.N., M.M.C., O.A., G.M.G. and J.H. have no competing interests.
Figures

References
-
- Ciardiello, F. & Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med.358, 1160–1174 (2008). - PubMed
-
- Lindeman, N. I. et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the college of american pathologists, the international association for the study of lung cancer, and the association for molecular pathology. Arch. Pathol. Lab. Med.142, 321–346 (2018). - PubMed
-
- NCCN clinical practice guidelines in oncology: non-small cell lung cancer. https://www.nccn.org/professionals/physiciangls/pdf/nscl.pdf (2024).
-
- Audibert, C. et al. Trends in the molecular diagnosis of lung cancer: results from an online market research survey. Friends of Cancer Research (2017); https://friendsofcancerresearch.org/wp-content/uploads/FINAL-2017-Friend...
-
- Robert, N. J. et al. Biomarker testing and tissue journey among patients with metastatic non-small cell lung cancer receiving first-line therapy in the US oncology network. Lung Cancer166, 197–204 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

