Plasma proteomics links brain and immune system aging with healthspan and longevity
- PMID: 40634782
- PMCID: PMC12353788
- DOI: 10.1038/s41591-025-03798-1
Plasma proteomics links brain and immune system aging with healthspan and longevity
Abstract
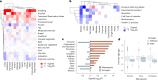
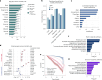
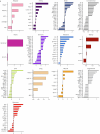
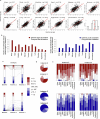
Plasma proteins derived from specific organs can estimate organ age and mortality, but their sensitivity to environmental factors and their robustness in forecasting onset of organ diseases and mortality remain unclear. To address this gap, we estimate the biological age of 11 organs using plasma proteomics data (2,916 proteins) from 44,498 individuals in the UK Biobank. Organ age estimates were sensitive to lifestyle factors and medications and were associated with future onset (within 17 years' follow-up) of a range of diseases, including heart failure, chronic obstructive pulmonary disease, type 2 diabetes and Alzheimer's disease. Notably, having an especially aged brain posed a risk of Alzheimer's disease (hazard ratio (HR) = 3.1) that was similar to carrying one copy of APOE4, the strongest genetic risk factor for sporadic Alzheimer's disease, whereas a youthful brain (HR = 0.26) provided protection that was similar to carrying two copies of APOE2, independent of APOE genotype. Accrual of aged organs progressively increased mortality risk (2-4 aged organs, HR = 2.3; 5-7 aged organs, HR = 4.5; 8+ aged organs, HR = 8.3), whereas youthful brains and immune systems were uniquely associated with longevity (youthful brain, HR = 0.60 for mortality risk; youthful immune system, HR = 0.58; youthful both, HR = 0.44). Altogether, these findings support the use of plasma proteins for monitoring of organ health and point to the brain and immune systems as key targets for longevity interventions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.W-C., H.S.-H.O. and J.R. are co-founders and scientific advisors of Teal Omics, Inc. and have received equity stakes. T.W-C. is co-founder of Vero Biosciences. The remaining authors declare no competing interests.
Figures






References
-
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell186, 243–278 (2023). - PubMed
-
- Tian, Y. E. et al. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat. Med.29, 1221–1231 (2023). - PubMed
-
- Sehgal, R. et al. Systems Age: a single blood methylation test to quantify aging heterogeneity across 11 physiological systems. Preprint at bioRxiv10.1101/2023.07.13.548904 (2023).
MeSH terms
Substances
Grants and funding
- R01 AG048076/AG/NIA NIH HHS/United States
- AG072255/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30 AG066515/AG/NIA NIH HHS/United States
- R21AG058859/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21 AG058859/AG/NIA NIH HHS/United States
- R01 AG072255/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- R01AG048076/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- UL1 TR003142/TR/NCATS NIH HHS/United States
- P50AG047366/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30AG066515/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

