Pharmacological inhibition of the cGAS-STING pathway suppresses microglia pyroptosis in sepsis-associated encephalopathy
- PMID: 40634978
- PMCID: PMC12243338
- DOI: 10.1186/s12974-025-03507-2
Pharmacological inhibition of the cGAS-STING pathway suppresses microglia pyroptosis in sepsis-associated encephalopathy
Abstract
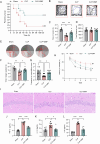
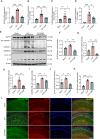
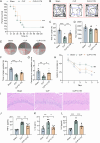
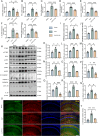
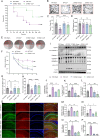
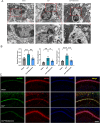
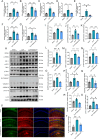
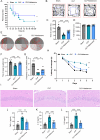
Sepsis-associated encephalopathy (SAE) presents significant challenges in clinical management due to its association with cognitive impairments and high mortality rates. This study aims to elucidate the molecular mechanisms underlying microglial pyroptosis and the stimulator of interferon genes (STING) signaling pathway in SAE, with a focus on identifying potential therapeutic targets. Employing the cecal ligation and puncture (CLP) model - the gold-standard polymicrobial sepsis model that faithfully replicates human sepsis progression - in C57BL/6J mice, we assessed the effects of various pharmacological agents, including the pyroptosis inhibitor dimethyl fumarate (DMF), the STING inhibitor C176, and the mitochondrial protectant idebenone, on cognitive and behavioral outcomes in SAE mice. Results indicated that DMF significantly prevented microglial pyroptosis, reduced inflammatory cytokine levels in the hippocampus, thereby enhancing survival rates and cognitive function. Additionally, inhibition of microglial pyroptosis through C176 effectively inhibited the STING signaling pathway, consequently reducing microglial pyroptosis and ameliorating behavioral symptoms associated with SAE. Furthermore, idebenone was observed to exert mitochondrial protection and inhibit STING-mediated pyroptosis of microglia, leading to an improvement in behavioral symptoms in the SAE model. In conclusion, our findings underscore the pivotal roles of pyroptosis and the STING signaling pathway in the pathophysiology of SAE, suggesting that targeting these mechanisms may provide promising therapeutic avenues for improving cognitive recovery in sepsis patients. Future studies should focus on elucidating the underlying molecular mechanisms and exploring their clinical viability in the management of SAE.
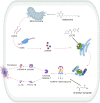
Graphical Abstract:
Supplementary Information: The online version contains supplementary material available at 10.1186/s12974-025-03507-2.
Keywords: Microglia; Pyroptosis; STING; Sepsis; Sepsis-associated encephalopathy.
Conflict of interest statement
Declarations. Ethical approval: All experimental protocols complied with international guidelines and were conducted under the supervision of the Ethics Committee for Animal Experiments of Fujian Medical University (Approval No. IACUC FJMU 2022 − 0880, approved on Dec. 21, 2022). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Hong Y, Chen P, Gao J, Lin Y, Chen L, Shang X. Sepsis-associated encephalopathy: from pathophysiology to clinical management. Int Immunopharmacol. 2023;124:110800. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

