Profiling of the tumor-associated microbiome in patients with hepatocellular carcinoma
- PMID: 40635009
- PMCID: PMC12243435
- DOI: 10.1186/s13099-025-00727-y
Profiling of the tumor-associated microbiome in patients with hepatocellular carcinoma
Abstract
Background: Tumor tissues have been shown to host a diverse array of bacteria, suggesting a link between the intratumoral microbiota and the development and progression of cancer. The aim of this explorative study was to perform microbiome analysis in liver tumor and to evaluate its relationship with cancer stage and survival outcome.
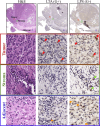
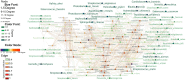
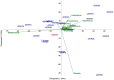
Results: We conducted an exploratory study on a cohort of 20 hepatocellular cancer patients from the SORAMIC trial. Patients were divided into curative and palliative groups according to treatment type (local ablation, alone or combined with systemic therapy). The V1-V2 regions of 16 S rRNA were sequenced starting from archival tissues. Amplicon Sequence Variants (ASVs) were taxonomically assigned to the upper (UGI) or lower (LGI) gastrointestinal tract. Bacteria were identified in both tumoral and non-tumoral tissues, showing higher diversity and correlation between diversity and shorter survival in the palliative group (S. aureus p < 0.05; B. parvula p < 0.01; A. chinensis p < 0.01). Both therapy groups were enriched with the genus Bacilli, including Streptococcus spp., Gemella haemolysans and Helicobacter pylori, commonly found in UGI. The results suggested that among palliative patients and those with shorter survival, G. haemolysans was more prevalent, while H. pylori was more often found in curative patients with longer survival. However none of the results were significantly different (p > 0.05). A higher microbiome biodiversity was associated with an increased number of lesions (Hoylesella, Agathobacter, Sphingobium, Cardiobacterium, Photobacterium and Serratia, all with p < 0.01).
Conclusions: The presence of bacteria, predominantly from communities of the UGI, suggests their translocation into liver tissue due to impaired barrier function of the upper gut or the ascending pathway along the biliary duct system. The intratumoral prevalence of bacteria with proinflammatory and oncogenic potential suggests their potential role in HCC pathomechanisms.
Keywords: Helicobacter pylori; Hepatocellular carcinoma; Interventional radiology; Liver; Microbiota.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The SORAMIC trial (EudraCT 2009-012576-27, NCT01126645) was approved by the institutional review boards of all 38 participating centers and was conducted according to the ethical principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all participants. Consent for publication: Not applicable. Competing interests: CS has received honoraria for lectures from Juvisé Pharmaceutics, Astellas and the Falk Foundation. CS has served in the Data Safety Monitoring Board or Advisory Board from Sanofi, Astella and Juvisé Pharmaceutics. CV has received consulting fees from IPSEN, AstraZeneca and Bayer and has received honoraria for lectures from IPSEN and Astra Zeneca.
Figures



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma.Cochrane Database Syst Rev. 2015 Jan 26;1(1):CD006745. doi: 10.1002/14651858.CD006745.pub3. Cochrane Database Syst Rev. 2015. PMID: 25620061 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Intratumoral microbiota: implications for cancer progression and treatment.Front Microbiol. 2025 Jul 28;16:1551515. doi: 10.3389/fmicb.2025.1551515. eCollection 2025. Front Microbiol. 2025. PMID: 40792257 Free PMC article. Review.
References
-
- Fernandes MR, Aggarwal P, Costa RGF, Cole AM, Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat Rev Cancer. 2022;22(12):703–22. - PubMed
-
- Battaglia TW, Mimpen IL, Traets JJH, van Hoeck A, Zeverijn LJ, Geurts BS, et al. A pan-cancer analysis of the Microbiome in metastatic cancer. Cell. 2024;187(9):2324–e3519. - PubMed
-
- Iida N, Mizukoshi E, Yamashita T, Yutani M, Seishima J, Wang Z, et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat Cancer. 2021;2(10):1039–54. - PubMed
-
- Kiran NS, Chatterjee A, Yashaswini C, Deshmukh R, Alsaidan OA, Bhattacharya S, et al. The Gastrointestinal mycobiome in inflammation and cancer: unraveling fungal dysbiosis, pathogenesis, and therapeutic potential. Med Oncol. 2025;42(6):195. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

