Efficacy and Safety of Glucagon-Like Peptide-1 Agonists for Psychiatric Symptoms: A Systematic Review
- PMID: 40635383
- PMCID: PMC12241715
- DOI: 10.1002/brb3.70661
Efficacy and Safety of Glucagon-Like Peptide-1 Agonists for Psychiatric Symptoms: A Systematic Review
Abstract
Background: Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have emerged as potential therapeutic options for psychiatric symptoms due to their effects on mood and behavior. However, their use has been primarily for metabolic diseases, and limited research has evaluated their psychiatric efficacy. This systematic review examined the impact of GLP-1 RAs on psychiatric symptoms, categorizing studies based on whether psychiatric outcomes were primary or secondary.
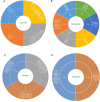
Methods: A comprehensive search through OVID databases from inception to November 2024 identified 26 studies (n = 3020), with 5 studies evaluating psychiatric symptoms as primary outcomes (e.g., substance use disorders) and 21 studies assessing secondary outcomes across various conditions. Additionally, 10 registered clinical trials were identified.
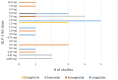
Results: Among the five studies targeting primary outcomes, exenatide and dulaglutide were investigated in cocaine use disorder, alcohol use disorder, and nicotine dependence. Exenatide 2 mg once-weekly demonstrated reductions in cocaine craving in two cases over 6 weeks in a case series study. However, mixed results were reported across studies, including no significant changes in a separate case series (n = 12, p = 0.46). Dulaglutide 1.5 mg weekly did not significantly affect smoking abstinence (RR = 0.87, p = 0.25) but reduced alcohol consumption by 29% in a secondary analysis. For studies assessing secondary outcomes, GLP-1 RAs showed variable effects. In mood disorders, liraglutide 1.8 mg/day significantly improved depression and anhedonia. In Type 2 diabetes, semaglutide improved diabetes-related quality of life (Cohen' and anxiety (Cohen's d = 0.48) compared to dulaglutide. Exenatide studies reported mixed results, with one randomized controlled trial (RCT) showing significant reductions in Beck's Depression Inventory (BDI) scores (Δ = -5.2). A total of nine RCTs were included in this review and the risk of bias was assessed using the JBI Critical Appraisal Checklist for RCTs. All included RCTs met the majority of criteria, indicating moderate-to-high methodological quality. Of the included RCTs, three reported a statistically significant effect on the primary psychiatric outcome, whereas six reported no significant effect.
Conclusion: GLP-1 RAs showed mixed and inconclusive effects on psychiatric symptoms. Although some studies suggested potential benefits, others reported null findings. Given the variability across trials, further research is needed to clarify their therapeutic potential.
Keywords: depression; glucagon‐like peptide‐1 (GLP‐1) receptor agonists; mental disorders; psychiatric disorders; systematic review.
© 2025 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
Jennifer Swainson has received honoraria for speaking or advisory roles from Abbvie, Bausch Health, Biron, Eisai, Idorsia, Janssen, Lundbeck, Novo Nordisk, and Otsuka. Rakesh Jetly is the CMO of Mydecine Innovation Group. Benjamin Dunkley is CSO at MYndspan Ltd, and has received funding from the Department of National Defence (Government of Canada), Canadian Institutes of Health Research, National Institutes of Health and MITACS. SMA has received honoraria from HLS therapeutics and Boehringer‐Ingelheim, Canada, and is supported in part by an Academic Scholar Award from the University of Toronto Department of Psychiatry. Venkat Bhat is supported by an Academic Scholar Award from the University of Toronto Department of Psychiatry and has received research support from the Canadian Institutes of Health Research, Brain & Behavior Foundation, Ontario Ministry of Health Innovation Funds, Royal College of Physicians and Surgeons of Canada, Department of National Defence (Government of Canada), New Frontiers in Research Fund, Associated Medical Services Inc. Healthcare, American Foundation for Suicide Prevention, Roche Canada, Novartis, and Eisai.
Figures
Similar articles
-
Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis.Diabetes Obes Metab. 2017 Apr;19(4):524-536. doi: 10.1111/dom.12849. Epub 2017 Feb 17. Diabetes Obes Metab. 2017. PMID: 27981757
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article.
-
Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis.Diabetes Obes Metab. 2017 Feb;19(2):228-238. doi: 10.1111/dom.12805. Epub 2016 Dec 5. Diabetes Obes Metab. 2017. PMID: 27717130 Free PMC article.
-
Pharmacotherapy for anxiety and comorbid alcohol use disorders.Cochrane Database Syst Rev. 2015 Jan 20;1(1):CD007505. doi: 10.1002/14651858.CD007505.pub2. Cochrane Database Syst Rev. 2015. PMID: 25601826 Free PMC article.
-
Efficacy, Safety, and Future of GLP-1 Receptor Agonists: A Systematic Literature Review and Meta-Analysis.Horm Metab Res. 2025 May;57(5):326-337. doi: 10.1055/a-2569-7315. Epub 2025 May 23. Horm Metab Res. 2025. PMID: 40409279
References
-
- Acar, A. S. , and Erbas O.. 2021. “2 Glucagon‐Like Peptide‐1 and Psychiatric Disorder.” Journal of Experimental and Basic Medical Sciences 2, no. 2: 106–115. 10.5606/jebms.2021.75645. - DOI
-
- Apperley, L. J. , Gait L., Erlandson‐Parry K., Laing P., and Senniappan S.. 2021. “Liraglutide Combined With Intense Lifestyle Modification in the Management of Obesity in Adolescents.” Journal of Pediatric Endocrinology and Metabolism 34, no. 5: 613–618. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

