Innate and Adaptive Immune Responses to Clinical Hyaluronic Acid Fillers
- PMID: 40635637
- PMCID: PMC12242719
- DOI: 10.1111/jocd.70292
Innate and Adaptive Immune Responses to Clinical Hyaluronic Acid Fillers
Abstract
Background: Crosslinked hyaluronic acid (HA)-based hydrogels are commonly used as dermal fillers where they interact with surrounding tissues including host stromal and immune cells. HA fillers are widely used for aesthetic applications, with products designed with varying properties depending on their indication. Although HA fillers have been demonstrated to have a strong biocompatibility profile, a small subset of patients' experiences delayed-onset adverse events hypothesized to be inflammatory and allergy-related outcomes such as delayed-onset hypersensitivity.
Aims: The overall goal of this study was to evaluate the innate and adaptive immune response to two clinically available HA filler formulations.
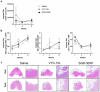
Methods: Using multiparametric flow cytometry, we characterized the immune response to Juvèderm Volbella (VYC-15 L) and Juvèderm Ultra 3 (SGD-30XP) in a murine quadricep muscle resection that enables implantation of larger volumes and exposure to muscle and adipose.
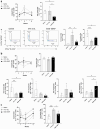
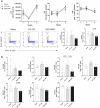
Results: Presence of the implanted HA filler increased recruitment of immune cells, specifically antigen presenting macrophages, eosinophils, and gamma-delta (γδ) T cells to the injury site compared to no implant (saline) controls. Comparing the two materials, VYC-15 L increased interleukin 17a (IL17a) production by lymphocyte subsets at the injury site and induced higher levels of circulating IgE relative to SGD-30XP and saline controls.
Conclusion: Overall, these results provide insights into the immune response to HA fillers and how different formulations may alter the immune outcomes.
Keywords: biomaterials; flow cytometry; hyaluronic acid; immune response.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Allergan funded this study and provided the materials for this study. J.H.E. is an inventor on intellectual property related to biological scaffolds and inhibiting fibrosis. J.H.E. holds equity in Unity Biotechnology and Aegeria Soft Tissue. J.H.E. is a member of the scientific advisory boards of Tessera Therapeutics, HapInScience, and Font Bio. J.H.E. is a consultant for Vericel. C.K.H. is an employee of Allergan Aesthetics, an AbbVie company, and owns stock and stock options in AbbVie.
Figures

References
-
- Fraser J. R. E., Laurent T. C., and Laurent U. B. G., “Hyaluronan: Its Nature, Distribution, Functions and Turnover,” Journal of Internal Medicine 242 (1997): 27–33. - PubMed
-
- Alijotas‐Reig J., Hindié M., Kandhaya‐Pillai R., and Miro‐Mur F., “Bioengineered Hyaluronic Acid Elicited a Nonantigenic T Cell Activation: Implications From Cosmetic Medicine and Surgery to Nanomedicine,” Journal of Biomedical Materials Research. Part A 95 (2010): 180–190. - PubMed
-
- Slevin M., Kumar S., and Gaffney J., “Angiogenic Oligosaccharides of Hyaluronan Induce Multiple Signaling Pathways Affecting Vascular Endothelial Cell Mitogenic and Wound Healing Responses *,” Journal of Biological Chemistry 277 (2002): 41046–41059. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

