Boosting Hydroxyl Radical Generation with Nitrogen Vacancy-Modified Carbon Nitride for Triggering Dual Damage of Cancer Nucleus DNA-Mitochondria against Hypoxic Tumors
- PMID: 40635718
- PMCID: PMC12239897
- DOI: 10.2147/IJN.S515726
Boosting Hydroxyl Radical Generation with Nitrogen Vacancy-Modified Carbon Nitride for Triggering Dual Damage of Cancer Nucleus DNA-Mitochondria against Hypoxic Tumors
Abstract
Introduction: Oral squamous cell carcinoma (OSCC) is a prevalent and deadly cancer, with over 350,000 new cases yearly. A hypoxic tumor microenvironment is the bottleneck of photodynamic therapy (PDT) and significantly weakens overall therapeutic efficacy.
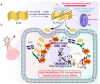
Methods: In this study, we introduce nitrogen vacancy-modified PCN (NV-PCN), a novel metal-free and O2-independent photosensitizer designed for PDT. NV-PCN targets Cal-27-induced OSCC by reducing highly expressed H2O2 in tumors to highly reactive •OH. This innovative approach aims to overcome the limitations posed by the hypoxic environment and enhance the effectiveness of PDT in treating OSCC.
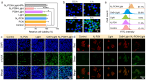
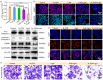
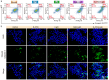
Results: The introduction of NV not only further improves the cell accessibility of PCN by increasing the content of -NH2 but also provides more reactive sites for H2O2 reduction and facilitates carrier separation. Under illumination, NV-PCN generates a burst of •OH around the nuclei and mitochondria of Cal-27 cells, which effectively kills the cells via synchronously leading to DNA damage and mitochondrial dysfunction. Compared to the conventional photosensitizer chlorin e6, NV-PCN-based PDT exhibits excellent anticancer performance in vitro and in vivo, highlighting its potential as a next-generation therapeutic agent.
Conclusion: Collectively, the high •OH-generation efficiency, strong anticancer activity, and overall safety of the O2-independent nanoparticle opens up new avenues for in-depth study on carbon nitride-based cancer PDT strategies. This work offers new hope for the effective treatment of OSCC and other challenging cancers.
Keywords: DNA-damage repair; hydroxyl radical; nitrogen vacancy; photodynamic therapy; polymeric carbon nitride.
© 2025 Zhang et al.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Tumor Microenvironment-Activated and ROS-Augmented Nanoplatform Amplified PDT against Colorectal Cancer through Impairing GPX4 To Induce Ferroptosis.ACS Appl Mater Interfaces. 2025 Jul 23;17(29):41586-41596. doi: 10.1021/acsami.5c05523. Epub 2025 Jul 9. ACS Appl Mater Interfaces. 2025. PMID: 40629874
-
Photosensitizing effectiveness of a novel chlorin-based photosensitizer for photodynamic therapy in vitro and in vivo.J Cancer Res Clin Oncol. 2014 Sep;140(9):1527-36. doi: 10.1007/s00432-014-1717-0. Epub 2014 May 27. J Cancer Res Clin Oncol. 2014. PMID: 24863752 Free PMC article.
-
Dual-activity nanozyme as an oxygen pump to alleviate tumor hypoxia and enhance photodynamic/ NIR-II photothermal therapy for sniping oral squamous cell carcinoma.Acta Biomater. 2024 Dec;190:476-487. doi: 10.1016/j.actbio.2024.10.018. Epub 2024 Oct 12. Acta Biomater. 2024. PMID: 39401597
-
The safety and efficiency of photodynamic therapy for the treatment of osteosarcoma: A systematic review of in vitro experiment and animal model reports.Photodiagnosis Photodyn Ther. 2022 Dec;40:103093. doi: 10.1016/j.pdpdt.2022.103093. Epub 2022 Aug 27. Photodiagnosis Photodyn Ther. 2022. PMID: 36031143
-
Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review.Int J Mol Sci. 2025 Mar 12;26(6):2528. doi: 10.3390/ijms26062528. Int J Mol Sci. 2025. PMID: 40141170 Free PMC article.
References
-
- Chen Q, Han L, Wu J, et al. A precise and intelligent nanomedicine for salivary adenoid cystic carcinoma treatment by combining antivascular photodynamic therapy and neuroinhibitory chemotherapy. Adv Funct Mater. 2024;34(13):2312732. doi: 10.1002/adfm.202312732 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

