Erosive Pustular Dermatosis and Amivantamab for Lung Cancer: A Case Report
- PMID: 40635880
- PMCID: PMC12240574
- DOI: 10.1159/000546616
Erosive Pustular Dermatosis and Amivantamab for Lung Cancer: A Case Report
Abstract
Introduction: Amivantamab is a monoclonal antibody against EGFR and MET receptors, indications for certain types of non-small cell lung cancer. Due to its mechanisms, cutaneous adverse effects are frequent and numerous.
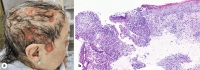
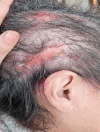
Case presentation: A 67-year-old woman with metastatic adenosquamous carcinoma, stage T3N2M1c, was treated with amivantamab after the first-line chemotherapy failed. Despite prophylactic oral tetracyclines, she developed severe erosive pustular dermatosis (EPD) affecting more than 50% of her scalp, forcing to cut short her hair to provide adequate local care.
Conclusion: EPD is an exceptional and severe adverse event of amivantamab, requiring oral steroids, tetracyclines and appropriate local care with antibiotic creams. Clinicians should be aware of this complication as early therapeutic intervention is mandatory to avoid deleterious consequences and spontaneous recurrences.
Keywords: Amivantamab; Case report; Erosive pustular dermatosis; Scalp erosion; Scalp ulceration.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Dermatologic Adverse Event Mitigation and Management Strategies with Amivantamab + Lazertinib Therapy for Advanced Non-Small Cell Lung Cancer: A Vodcast.Target Oncol. 2025 Jun 21. doi: 10.1007/s11523-025-01163-3. Online ahead of print. Target Oncol. 2025. PMID: 40544186
-
Minocycline for acne vulgaris: efficacy and safety.Cochrane Database Syst Rev. 2003;(1):CD002086. doi: 10.1002/14651858.CD002086. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD002086. doi: 10.1002/14651858.CD002086.pub2. PMID: 12535427 Updated.
-
Intracavity lavage and wound irrigation for prevention of surgical site infection.Cochrane Database Syst Rev. 2017 Oct 30;10(10):CD012234. doi: 10.1002/14651858.CD012234.pub2. Cochrane Database Syst Rev. 2017. PMID: 29083473 Free PMC article.
References
-
- Liao Z, Luo C, Huang Y, Jiang Z, Wei H, Wang Y. Evaluation of the safety profile of amivantamab based on real-world evidence: a call to vigilance. Expert Opin Drug Saf. 2025:1–10. - PubMed
-
- Basse C, Chabanol H, Bonte PE, Fromantin I, Girard N. Management of cutaneous toxicities under amivantamab (anti MET and anti EGFR bispecific antibody) in patients with metastatic non-small cell lung cancer harboring EGFR Exon20ins: towards a proactive, multidisciplinary approach. Lung Cancer. 2022;173:116–23. - PubMed
-
- Nikolaou V, Gerochristou M, Sgontzou T, Silaidi C, Stratigos A. Beneath the surface: novel insights into Amivantamab-induced acneiform rash. Eur J Cancer. 2024;208:114198. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

