Cost-effectiveness analysis of cadonilimab plus bevacizumab and chemotherapy for persistent, recurrent, or metastatic cervical cancer
- PMID: 40636104
- PMCID: PMC12237631
- DOI: 10.3389/fimmu.2025.1594786
Cost-effectiveness analysis of cadonilimab plus bevacizumab and chemotherapy for persistent, recurrent, or metastatic cervical cancer
Abstract
Objective: The aim of this study is to investigate the cost-effectiveness of cadonilimab plus bevacizumab and chemotherapy in the first-line treatment for patients with persistent, recurrent, or metastatic cervical cancer from a healthcare system perspective in China.
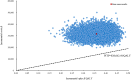
Methods: A partitioned survival model was established to estimate the total costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) over a 10-year time horizon. Clinical data was sourced from the COMPASSION-16 trial; direct medical costs and utilities were obtained from a public drug bidding database and published literature. The robustness of the model was assessed via scenario, one-way and probabilistic sensitivity analyses.
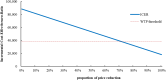
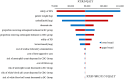
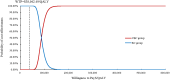
Results: Cadonilimab plus bevacizumab and chemotherapy yielded an additional cost of $31,654.02, with an additional QALY of 0.36, resulted in an ICER of $88,533.51/QALY compared with bevacizumab and chemotherapy. Utility values of progression-free survival (PFS), patient weight and price of cadonilimab were the most influential parameter on ICER. The probability of cadonilimab plus bevacizumab and chemotherapy being cost-effective was 0% at the WTP threshold of $38,042.49 per QALY. When the price of cadonilimab reduced by 72%, cadonilimab plus bevacizumab and chemotherapy would represent an economically viable treatment regime.
Conclusion: Cadonilimab plus bevacizumab and chemotherapy may not be a cost-effective option as the first-line treatment in persistent, recurrent, or metastatic cervical cancer.
Keywords: bevacizumab; cadonilimab; cervical cancer; chemotherapy; cost-effectiveness.
Copyright © 2025 Wang, Liu, Wang, Dou and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cost-effectiveness analysis of cadonilimab plus chemotherapy as a first-line treatment option in HER-2-negative advanced gastric cancer.Front Public Health. 2025 Jul 21;13:1644176. doi: 10.3389/fpubh.2025.1644176. eCollection 2025. Front Public Health. 2025. PMID: 40761936 Free PMC article.
-
Cost-effectiveness of enzalutamide with androgen-deprivation therapy (ADT) versus ADT alone for the treatment of high-risk biochemically recurrent non-metastatic castration-sensitive prostate cancer in Canada.J Med Econ. 2025 Dec;28(1):766-777. doi: 10.1080/13696998.2025.2503660. Epub 2025 May 23. J Med Econ. 2025. PMID: 40395149
-
Cadonilimab plus chemotherapy as first-line treatment for persistent, recurrent, or metastatic cervical cancer: a cost-effectiveness analysis.Front Immunol. 2025 Apr 3;16:1562875. doi: 10.3389/fimmu.2025.1562875. eCollection 2025. Front Immunol. 2025. PMID: 40248696 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. (2017) 390:1654–63. doi: 10.1016/S0140-6736(17)31607-0 - DOI - PMC - PubMed

