Organ of Corti macrophages: a distinct group of cochlear macrophages with potential roles in supporting cell degeneration and survival
- PMID: 40636110
- PMCID: PMC12237686
- DOI: 10.3389/fimmu.2025.1617146
Organ of Corti macrophages: a distinct group of cochlear macrophages with potential roles in supporting cell degeneration and survival
Abstract
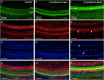
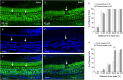
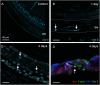
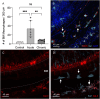
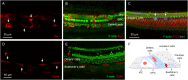
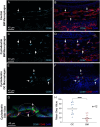
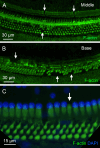
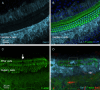
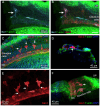
Macrophages are the primary immune cells in the cochlea, essential for maintaining cochlear homeostasis and orchestrating inflammatory responses to pathological events. Although these cells have been found in various parts of the cochlea, their presence in the organ of Corti, a critical structure for acoustic sensing, remains poorly understood. The present study was designed to examine macrophage responses to ototoxic drug-induced cochlear damage and age-related cochlear degeneration, with a particular focus on the pathological conditions that trigger macrophage recruitment into the organ of Corti. We used a model of ototoxicity induced by cyclodextrin, a cyclic oligosaccharide known for its ability to induce rapid sensory cell damage at high doses. Cochlear tissues were collected for macrophage assessment. Multiple protein markers, including CD45, Iba1, galectin-3, CD68, myosin 7α, and IFIT3, were used to label sensory cells, macrophages, and supporting cells in cochlear sensory epithelia. Consistent with previous reports, our study confirms that macrophages are absent from the organ of Corti in mouse cochleae under normal conditions and during the acute phase of cochlear damage. However, macrophages enter the organ of Corti during the chronic phase of cochlear pathogenesis. These macrophages exhibit an activated state and display a distinct functional profile compared to macrophages outside the organ of Corti. Importantly, we demonstrate that the organ of Corti macrophage activity is not directly related to the process of sensory cell degradation. Instead, their activity is associated with supporting cell pathogenesis. Moreover, our study shows that the organ of Corti macrophages are also present in aging mouse cochleae. Collectively, our findings reveal the conditions that lead to macrophage recruitment into the organ of Corti and their involvement in supporting cell survival and degeneration. These findings provide valuable insights for future strategies focused on modulating macrophage activity to reduce tissue damage and promote repair in the cochlea following injury.
Keywords: aging; cochlea; galectin-3; inflammation; macrophage; organ of Corti; ototoxicity; supporting cells.
Copyright © 2025 Ye, Zhang, Ding, Chen, Adler, Sharaf and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

