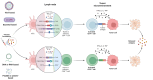
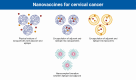
Nanovaccines against Cervical Cancer: Reliable Strategies to Circumvent Limitations of Traditional Therapeutic Vaccines
- PMID: 40636309
- PMCID: PMC12235371
- DOI: 10.34172/apb.43712
Nanovaccines against Cervical Cancer: Reliable Strategies to Circumvent Limitations of Traditional Therapeutic Vaccines
Abstract
Cervical cancer ranks fourth in terms of diagnosis and cancer-related deaths in women worldwide. Despite the approval of prophylactic vaccines against cervical cancers, these vaccines are not able to eradicate the existing ones. Therefore, various platforms have been developed to design therapeutic vaccines against cervical cancers, including DNA/RNA-based, protein/peptide-based, vector-based, and cell-based platforms. Despite the advantages of each platform, therapeutic vaccines have displayed limited clinical benefit in patients with cervical cancer, which is partially associated with inefficient delivery of vaccine components. To address these issues, different nanoplatforms have been developed to carry cellular or molecular components of vaccines to target cells and lymphoid tissues, thus promoting the durability and potency of immune responses against tumor cells and antigens besides decreasing side effects. Moreover, nanoparticles (NPs), as adjuvants and/or carriers, provide other advantages, including sufficient antigen loading and uptake by antigen-presenting cells (APCs), adaptable antigen presentation, high immunogenicity, high stability, increased lymph node retention, and precise targeting. Thus, nanovaccines also lead us to design and develop personalized vaccines against cervical cancer. Here, we discuss platforms that have been used in clinical trials for the treatment of cervical cancer, their advantages and disadvantages, platforms for developing nanovaccines, and how they improve the therapeutic efficacy of vaccines.
Keywords: Cervical cancer; Immune response; Nanotechnology; Nanovaccine; Therapeutic vaccine.
© 2025 The Author (s).
Conflict of interest statement
The authors declare no conflict of interest.
Similar articles
-
Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors.Cochrane Database Syst Rev. 2018 May 9;5(5):CD009069. doi: 10.1002/14651858.CD009069.pub3. Cochrane Database Syst Rev. 2018. PMID: 29740819 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Adjuvanted RNA Origami-A Tunable Peptide Assembly Platform for Constructing Cancer Nanovaccines.Vaccines (Basel). 2025 May 25;13(6):560. doi: 10.3390/vaccines13060560. Vaccines (Basel). 2025. PMID: 40573891 Free PMC article.
-
Nanotechnology-driven enhancement and modulation of immune responses in monkeypox and respiratory syncytial virus nanovaccine research.Colloids Surf B Biointerfaces. 2025 Oct;254:114829. doi: 10.1016/j.colsurfb.2025.114829. Epub 2025 May 29. Colloids Surf B Biointerfaces. 2025. PMID: 40450846 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous