Prevention of Clostridium Difficile Infection Among Hospitalized Elderly Patients Using Torani (Fermented Rice Water) and Xylitol Mixture Drink: The Study Protocol of an Open-Label Randomized Controlled Trial
- PMID: 40636622
- PMCID: PMC12240546
- DOI: 10.7759/cureus.85635
Prevention of Clostridium Difficile Infection Among Hospitalized Elderly Patients Using Torani (Fermented Rice Water) and Xylitol Mixture Drink: The Study Protocol of an Open-Label Randomized Controlled Trial
Abstract
Background and objectives: Clostridium difficile infections (CDI) among elderly individuals are common. Antibiotic use and gut dysbiosis are major contributors to CDI. Probiotics and prebiotics help prevent CDI by addressing dysbiosis. This study aims to document the effect of the combination of probiotics from Torani, a traditional fermented food (rich in Lactobacillus species), and prebiotics (xylitol) on CDI among elderly hospitalized individuals.
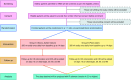
Methods: This two-arm, open-label, randomized controlled trial (RCT) will be conducted at a tertiary care hospital in Odisha, India. The eligible elderly hospitalized participants will be randomized into two groups in a 1:1 ratio to receive 350 ml of either Torani-xylitol mixture or plain water once daily for 14 days. The data on sociodemography, clinical, and laboratory tests, antibiotics, and other medications used shall be recorded from their case sheets. Stool samples or rectal swabs will be collected on days 0 and 15 or at discharge/death/referral for CD isolation. A stool or rectal swab sample will be collected for any suspected CDI during the hospitalization. Follow-up contacts will be made on days 30 (+7), 60 (+7), and 90 (+7) to address any illness/infection, medication use, hospitalization, blood tests, and dietary practices.
Results: As we have not started the study, we do not have any observations yet.
Conclusions: This RCT shall document the effect of the Torani-xylitol mixture on CDI prevention and CD colonization among hospitalized elderly individuals. If found effective, it can promote using beneficial fermented foods to prevent CDI, reducing morbidity, mortality, hospitalization rate, and duration, cost of therapy, and bed occupancy.
Keywords: clostridioides difficile infection; fermented diet; gut dysbiosis; gut-microbiota; microbial colonization; prebiotics; probiotics; short chain fatty acids; torani; xylitol.
Copyright © 2025, Pathi et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. INSTITUTIONAL ETHICS COMMITTEE KALINGA INSTITUTE OF MEDICAL SCIENCES (KIMS) issued approval KIIT/KIMS/IEC/1753/2024. The Institutional Ethics Committee of KIMS, Bhubaneswar (KIIT/KIMS/IEC/1753/2024), approved the study protocol on March 25, 2024. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Epidemiology of Clostridioides difficile infection in hospitalized patients in Spain: An eight-year review (2012-2019) Asensio Á, Vallejo-Plaza A, Parra LM, et al. Enferm Infecc Microbiol Clin (Engl Ed) 2022;40:125–130. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials